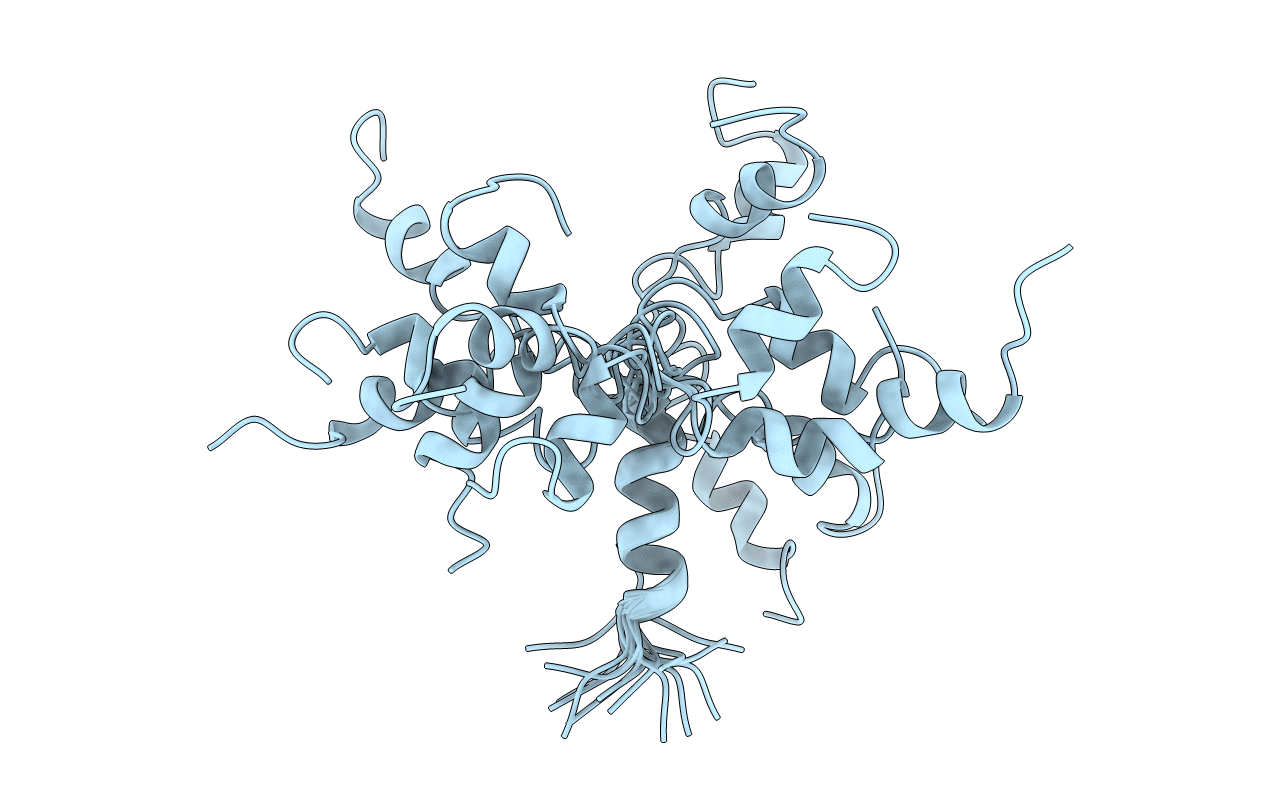
Deposition Date
2002-05-02
Release Date
2002-11-27
Last Version Date
2023-12-27
Entry Detail
PDB ID:
1IWC
Keywords:
Title:
TFE-induded structure of the N-terminal domain of pig gastric H/K-ATPase
Method Details:
Experimental Method:
Conformers Calculated:
30
Conformers Submitted:
15
Selection Criteria:
structures with the lowest energy