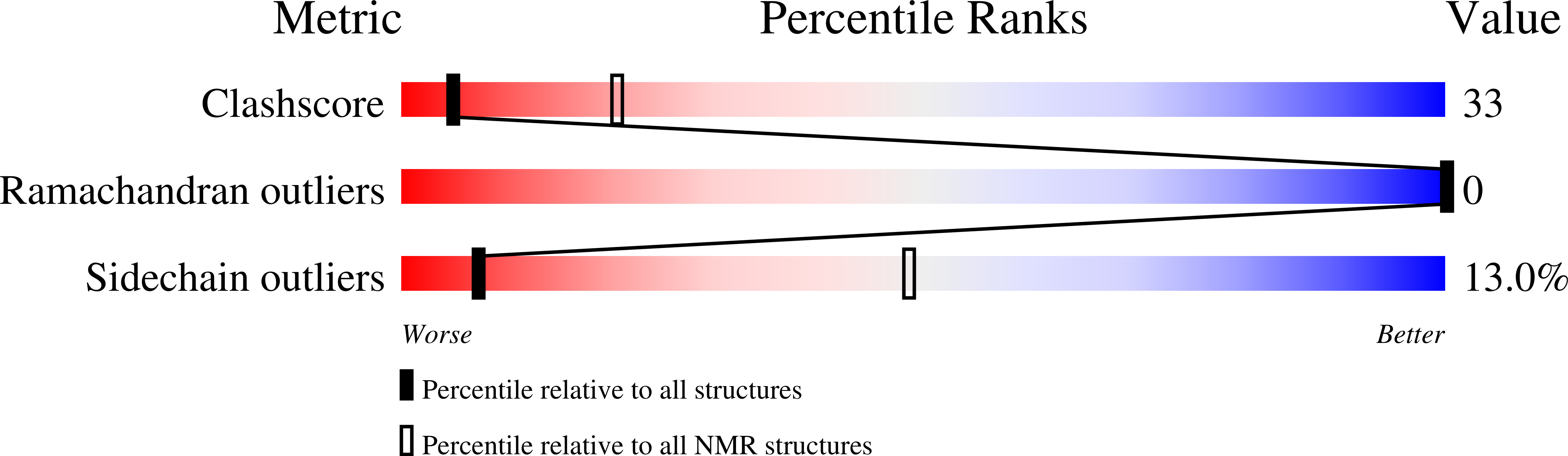
Deposition Date
2001-10-25
Release Date
2003-02-11
Last Version Date
2023-12-27
Entry Detail
PDB ID:
1IRZ
Keywords:
Title:
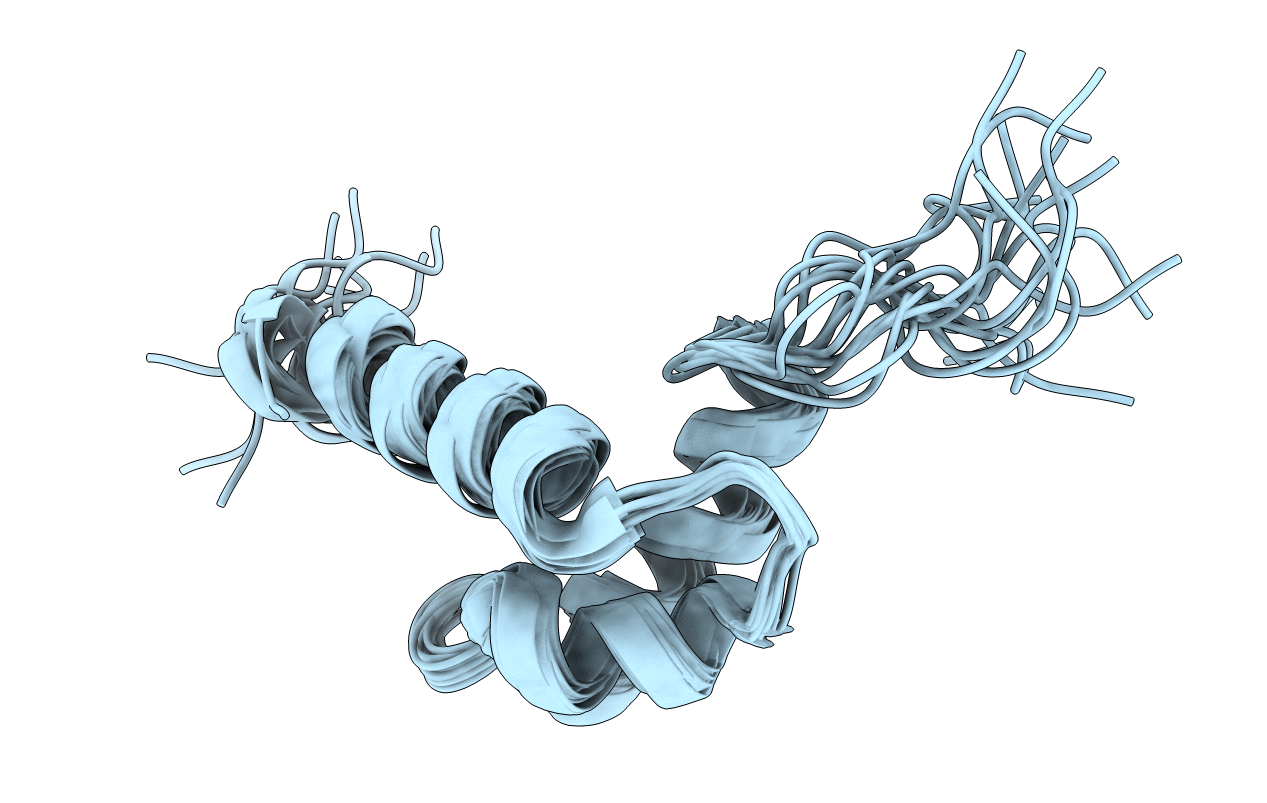
Solution structure of ARR10-B belonging to the GARP family of plant Myb-related DNA binding motifs of the Arabidopsis response regulators
Biological Source:
Source Organism(s):
Arabidopsis thaliana (Taxon ID: 3702)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
15
Selection Criteria:
structures with lowest energies