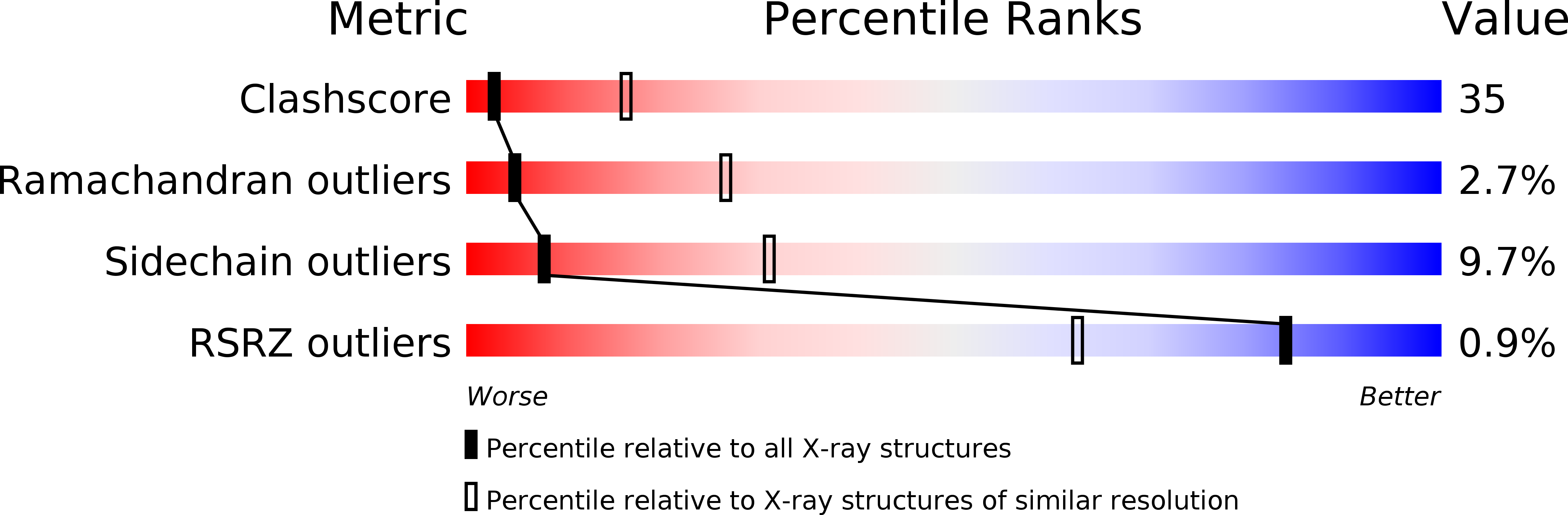Deposition Date
2001-01-10
Release Date
2001-03-12
Last Version Date
2023-12-27
Entry Detail
PDB ID:
1IO4
Keywords:
Title:
CRYSTAL STRUCTURE OF RUNX-1/AML1/CBFALPHA RUNT DOMAIN-CBFBETA CORE DOMAIN HETERODIMER AND C/EBPBETA BZIP HOMODIMER BOUND TO A DNA FRAGMENT FROM THE CSF-1R PROMOTER
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Mus musculus (Taxon ID: 10090)
Mus musculus (Taxon ID: 10090)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.00 Å
R-Value Free:
0.29
R-Value Work:
0.24
Space Group:
C 2 2 21