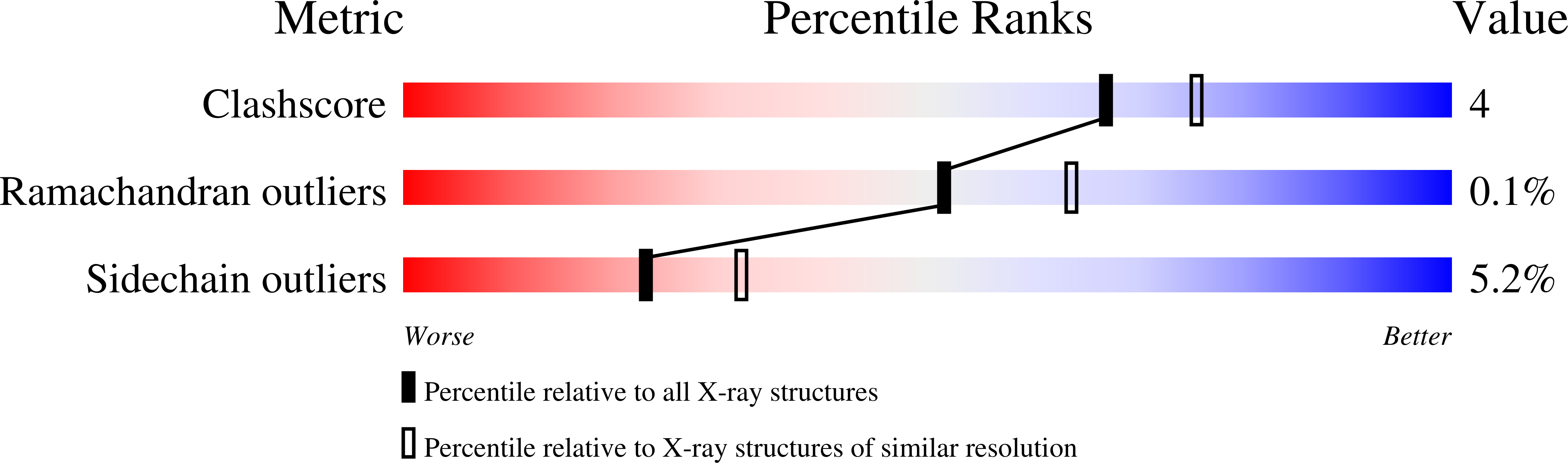
Deposition Date
1995-10-12
Release Date
1996-03-08
Last Version Date
2024-10-09
Entry Detail
PDB ID:
1ILU
Keywords:
Title:
X-RAY CRYSTAL STRUCTURE THE TWO SITE-SPECIFIC MUTANTS ILE7SER AND PHE110SER OF AZURIN FROM PSEUDOMONAS AERUGINOSA
Biological Source:
Source Organism(s):
Pseudomonas aeruginosa (Taxon ID: 287)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.30 Å
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 1 21 1