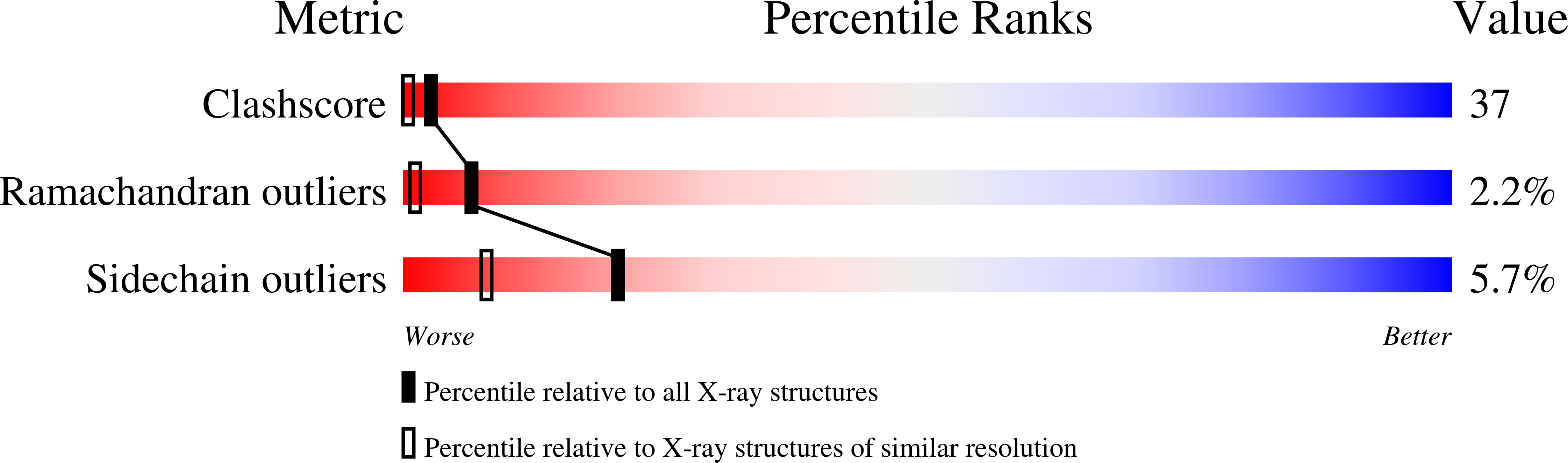Deposition Date
2001-04-24
Release Date
2001-08-08
Last Version Date
2024-10-16
Entry Detail
PDB ID:
1IJ3
Keywords:
Title:
GCN4-pVSL Coiled-coil trimer with Serine at the a(16) position
Method Details:
Experimental Method:
Resolution:
1.80 Å
R-Value Free:
0.26
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 1