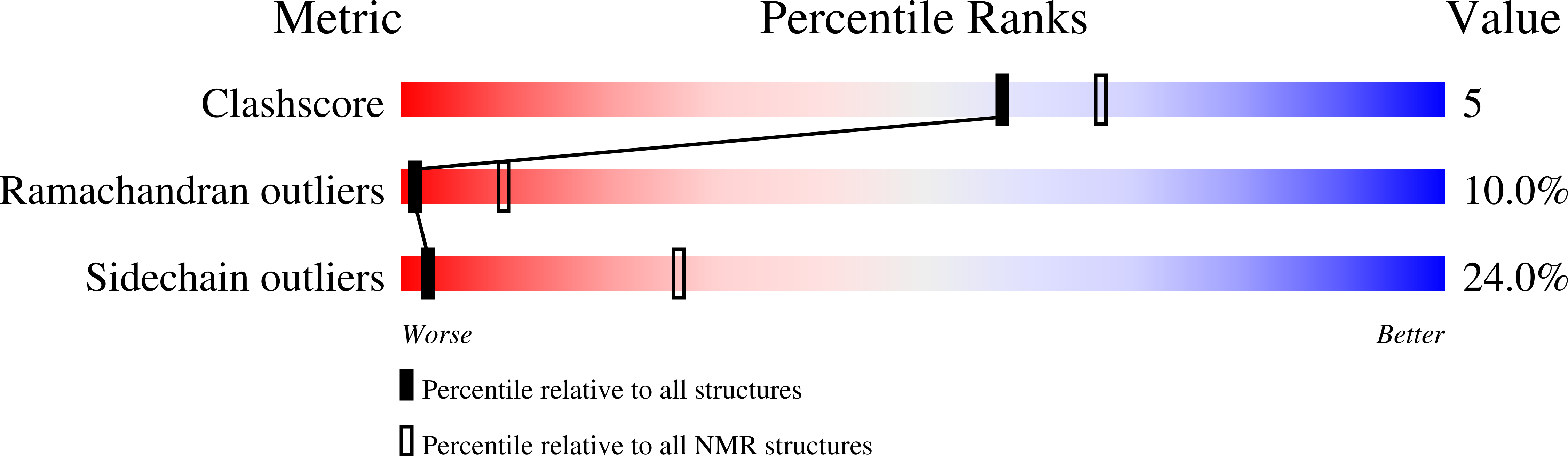
Deposition Date
2001-04-04
Release Date
2001-06-27
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1ID8
Keywords:
Title:
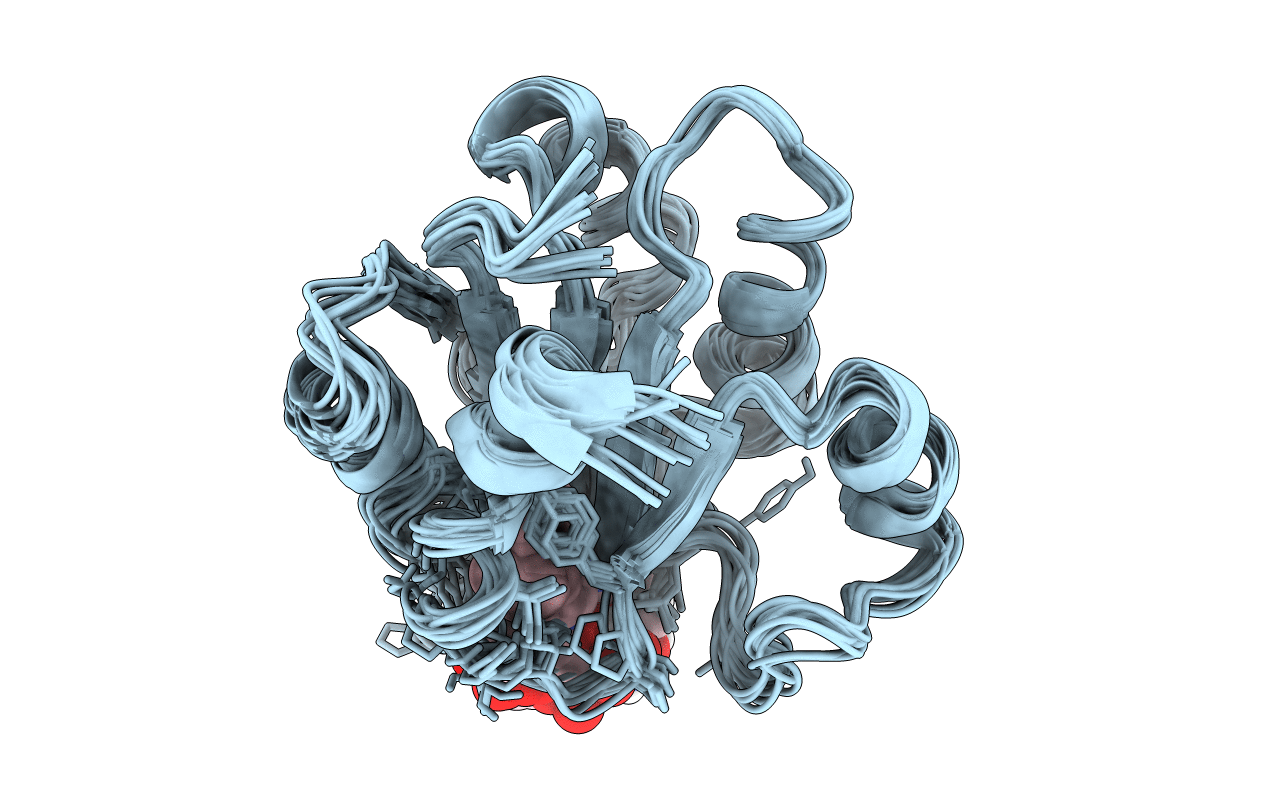
NMR STRUCTURE OF GLUTAMATE MUTASE (B12-BINDING SUBUNIT) COMPLEXED WITH THE VITAMIN B12 NUCLEOTIDE
Biological Source:
Source Organism(s):
Clostridium tetanomorphum (Taxon ID: 1553)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
30
Conformers Submitted:
15
Selection Criteria:
structures with the least restraint violations