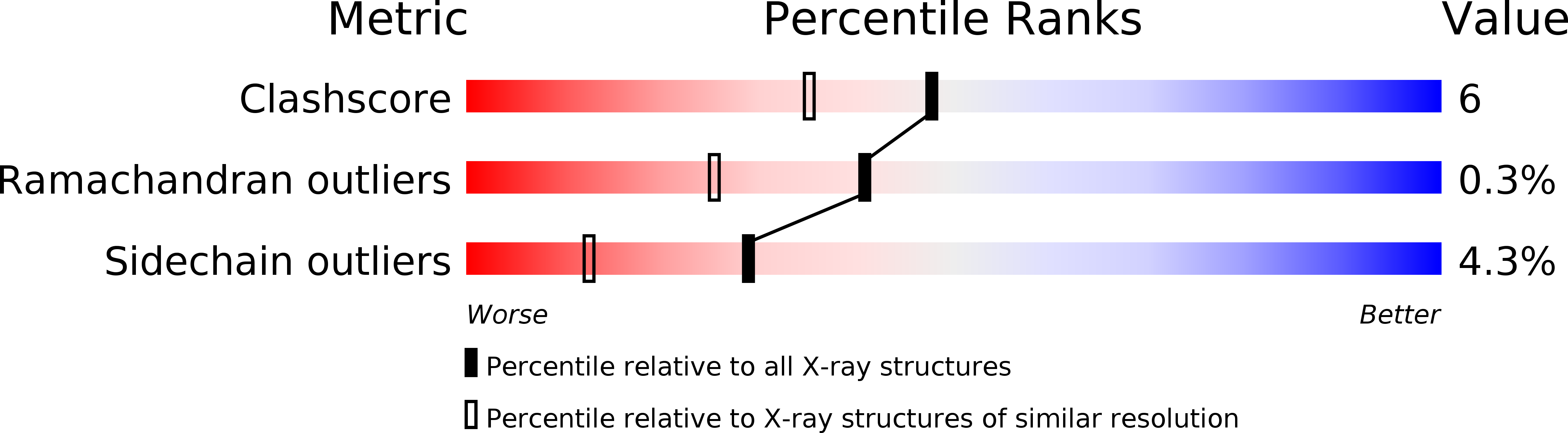
Deposition Date
2001-03-22
Release Date
2001-04-11
Last Version Date
2023-08-09
Entry Detail
PDB ID:
1IA3
Keywords:
Title:
Candida albicans dihydrofolate reductase complex in which the dihydronicotinamide moiety of dihydro-nicotinamide-adenine-dinucleotide phosphate (NADPH) is displaced by 5-[(4-TERT-BUTYLPHENYL)SULFANYL]-2,4-QUINAZOLINEDIAMINE (GW995)
Biological Source:
Source Organism(s):
Candida albicans (Taxon ID: 5476)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.78 Å
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 1 21 1