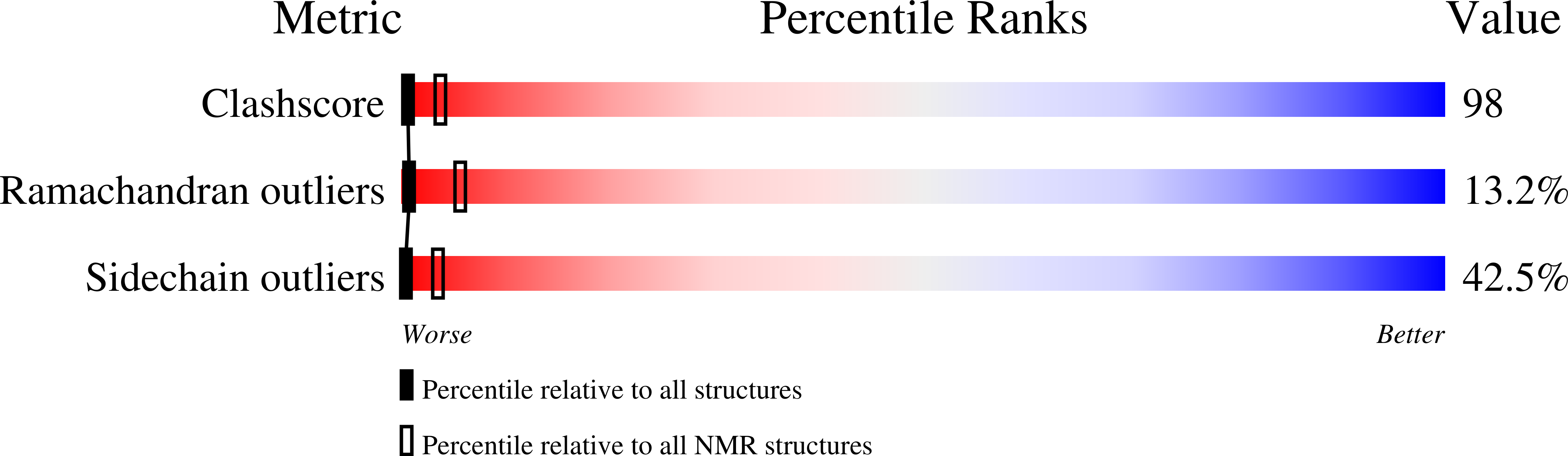Deposition Date
2001-02-13
Release Date
2001-12-12
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1I35
Keywords:
Title:
SOLUTION STRUCTURE OF THE RAS-BINDING DOMAIN OF THE PROTEIN KINASE BYR2 FROM SCHIZOSACCHAROMYCES POMBE
Biological Source:
Source Organism(s):
Schizosaccharomyces pombe (Taxon ID: 4896)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
10
Selection Criteria:
The submitted conformer models are the 10 structures with the lowest total energy