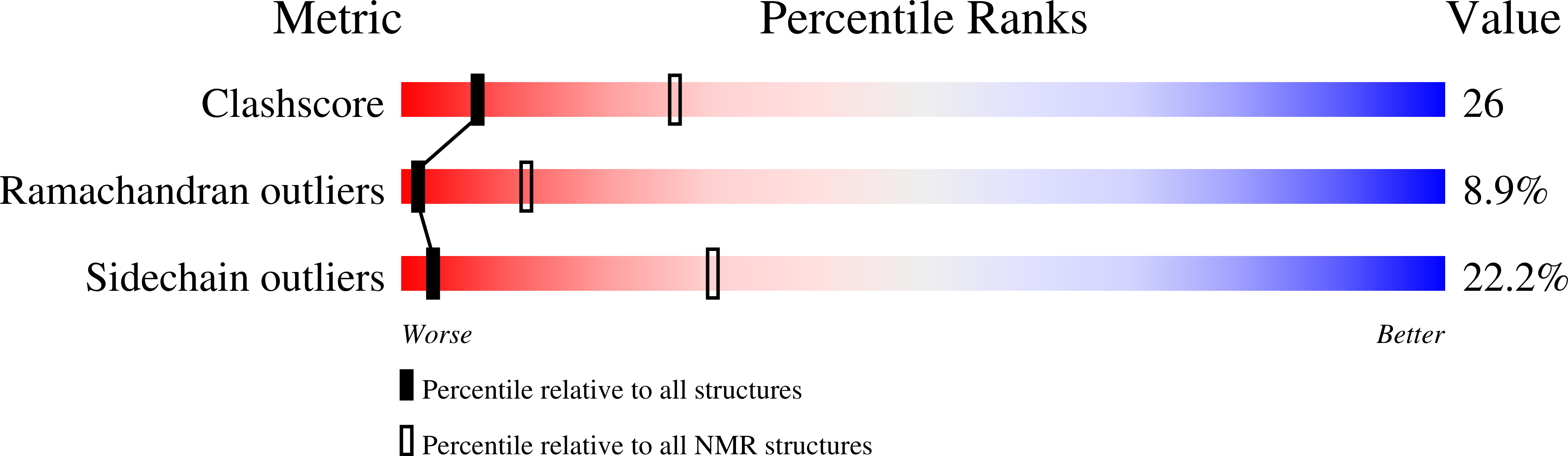
Deposition Date
2001-02-12
Release Date
2002-02-12
Last Version Date
2024-11-20
Entry Detail
PDB ID:
1I2V
Keywords:
Title:
NMR SOLUTION STRUCTURES OF AN ANTIFUNGAL AND ANTIBACTERIAL MUTANT OF HELIOMICIN
Biological Source:
Source Organism(s):
Heliothis virescens (Taxon ID: 7102)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
40
Conformers Submitted:
18
Selection Criteria:
structures with acceptable covalent geometry, structures with favorable non-bond energy, structures with the least restraint violations, structures with the lowest energy, target function