Deposition Date
1994-05-04
Release Date
1994-07-31
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1HYT
Keywords:
Title:
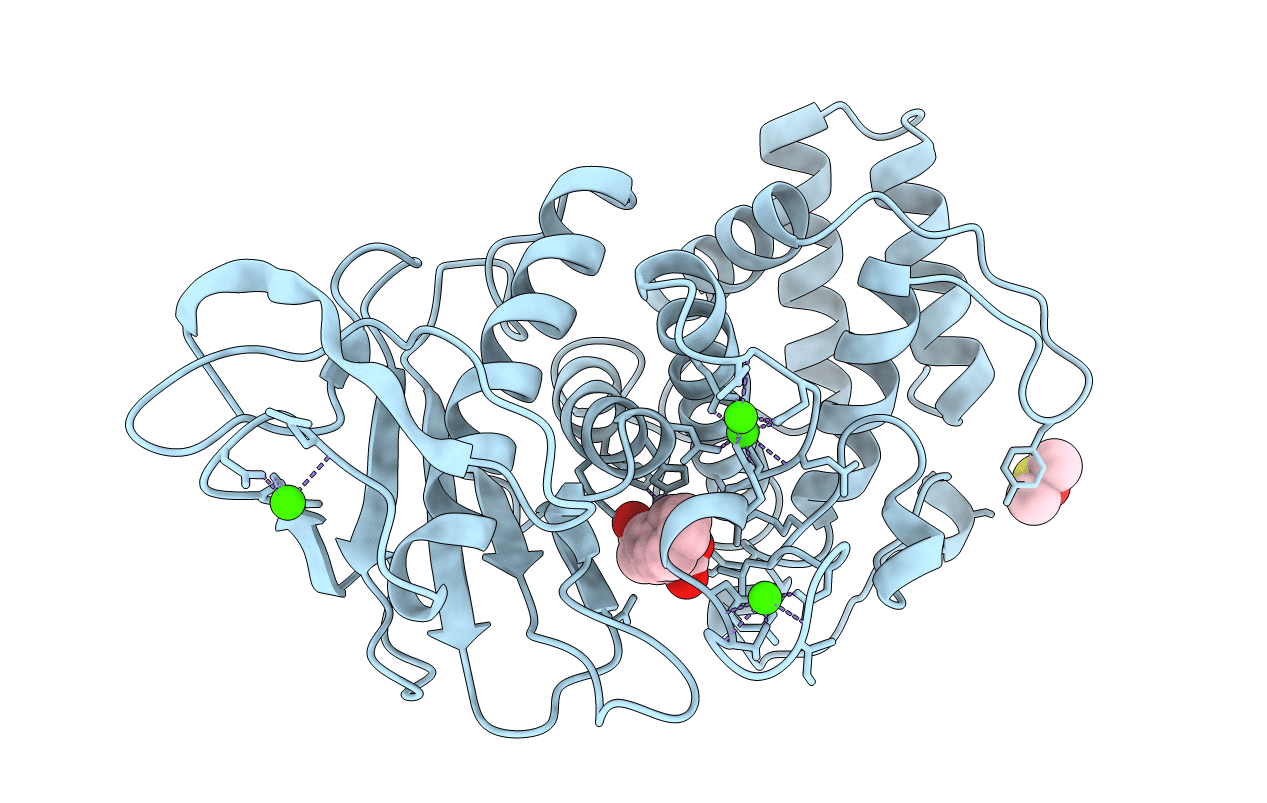
RE-DETERMINATION AND REFINEMENT OF THE COMPLEX OF BENZYLSUCCINIC ACID WITH THERMOLYSIN AND ITS RELATION TO THE COMPLEX WITH CARBOXYPEPTIDASE A
Biological Source:
Source Organism(s):
Bacillus thermoproteolyticus (Taxon ID: 1427)
Method Details:
Experimental Method:
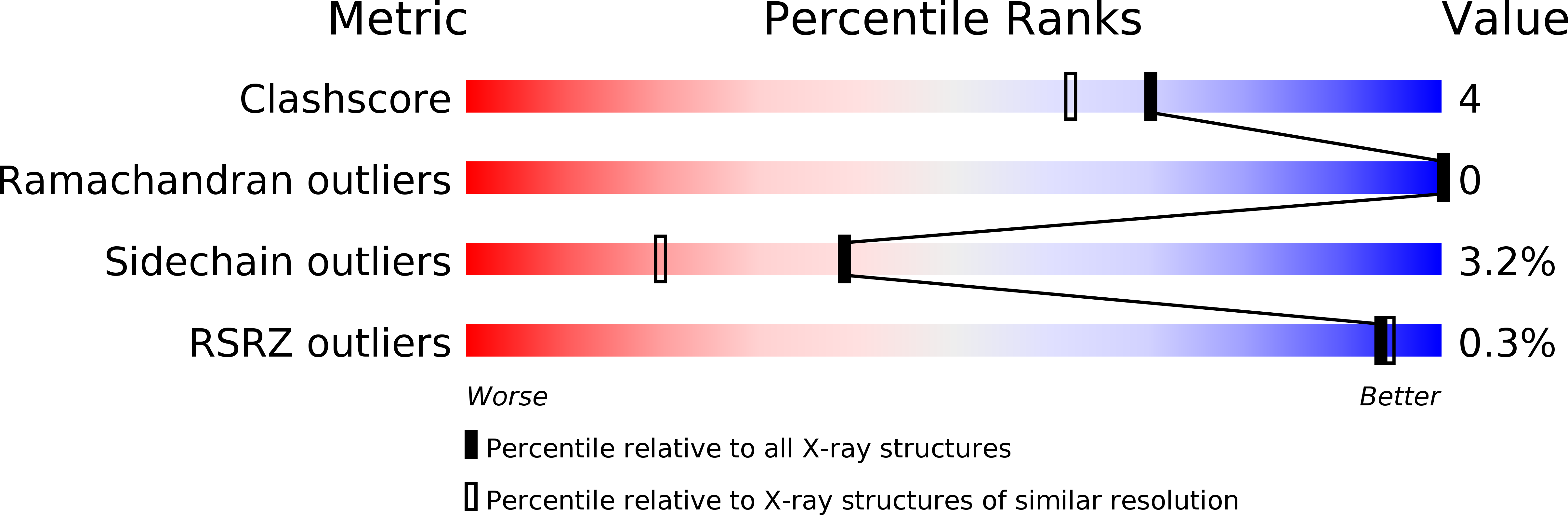
Resolution:
1.70 Å
R-Value Observed:
0.15
Space Group:
P 61 2 2