Deposition Date
2001-01-15
Release Date
2001-03-21
Last Version Date
2024-10-16
Entry Detail
PDB ID:
1HXI
Keywords:
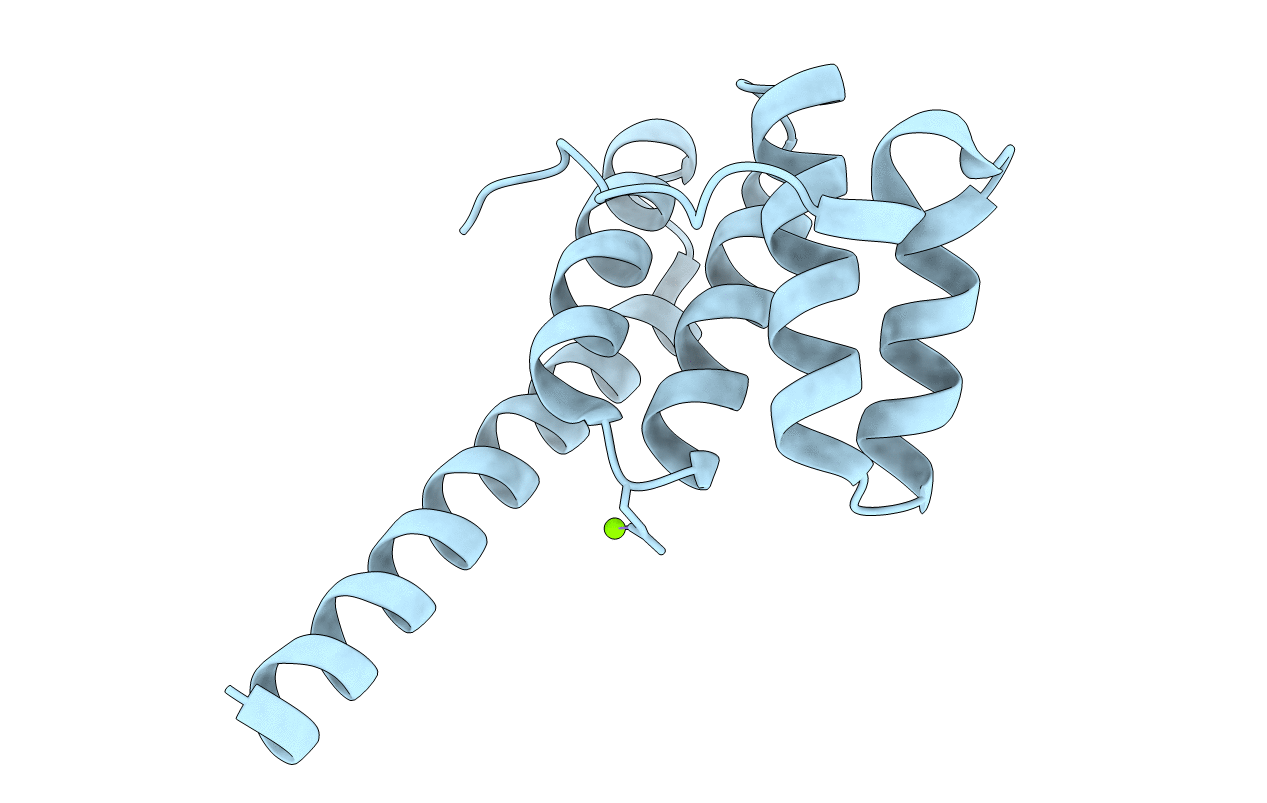
Title:
AN UNEXPECTED EXTENDED CONFORMATION FOR THE THIRD TPR MOTIF OF THE PEROXIN PEX5 FROM TRYPANOSOMA BRUCEI
Biological Source:
Source Organism:
Trypanosoma brucei (Taxon ID: 5691)
Host Organism:
Method Details:
Experimental Method:
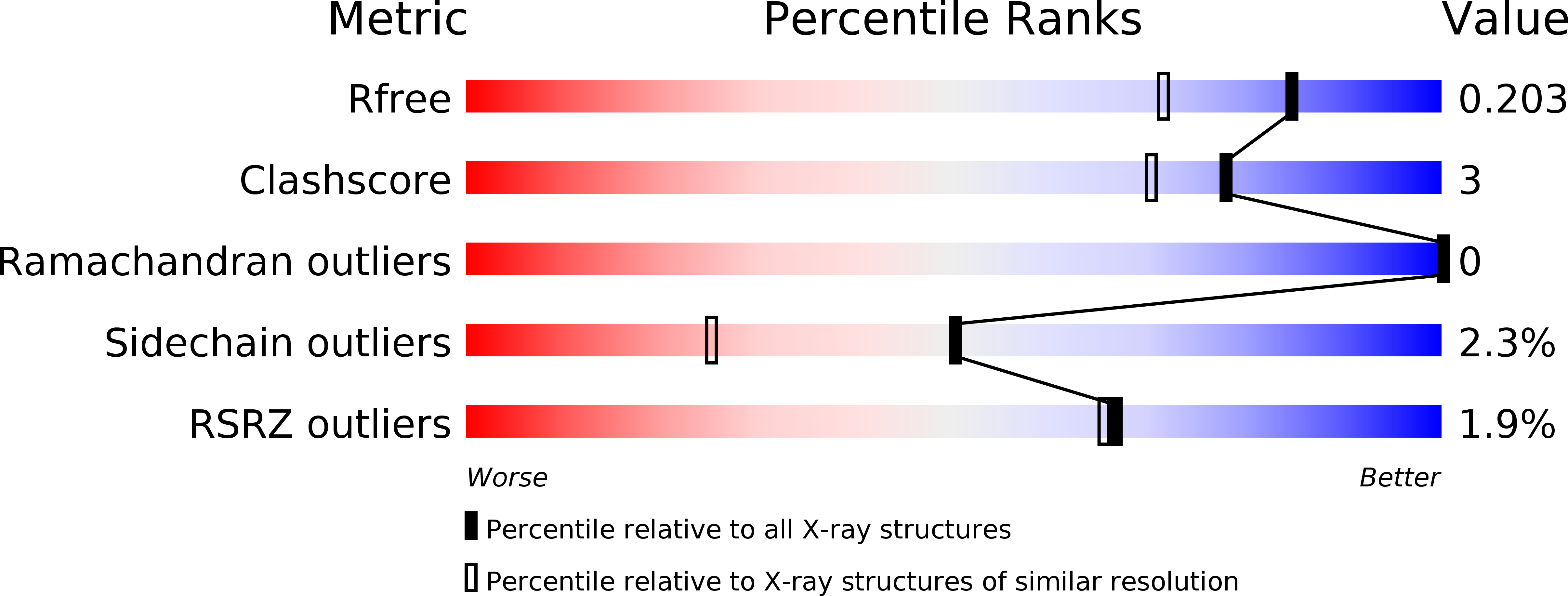
Resolution:
1.60 Å
R-Value Free:
0.22
R-Value Work:
0.18
R-Value Observed:
0.19
Space Group:
P 65