Deposition Date
2000-12-28
Release Date
2001-05-02
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1HT4
Keywords:
Title:
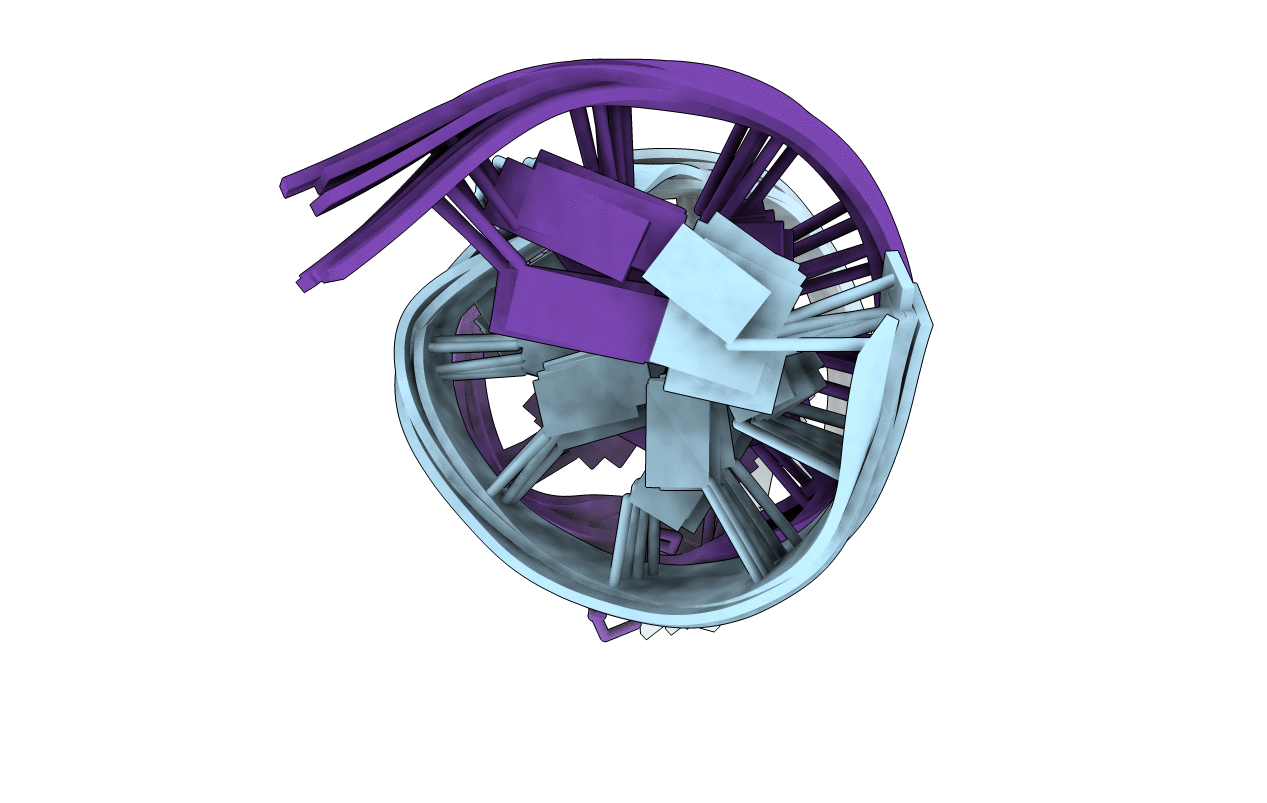
SOLUTION STRUCTURE OF A BISTRAND ABASIC SITE LESION STAGGERED IN A 3'-ORIENTATION.
Method Details:
Experimental Method:
Conformers Calculated:
30
Conformers Submitted:
6
Selection Criteria:
structures with the least restraint violations