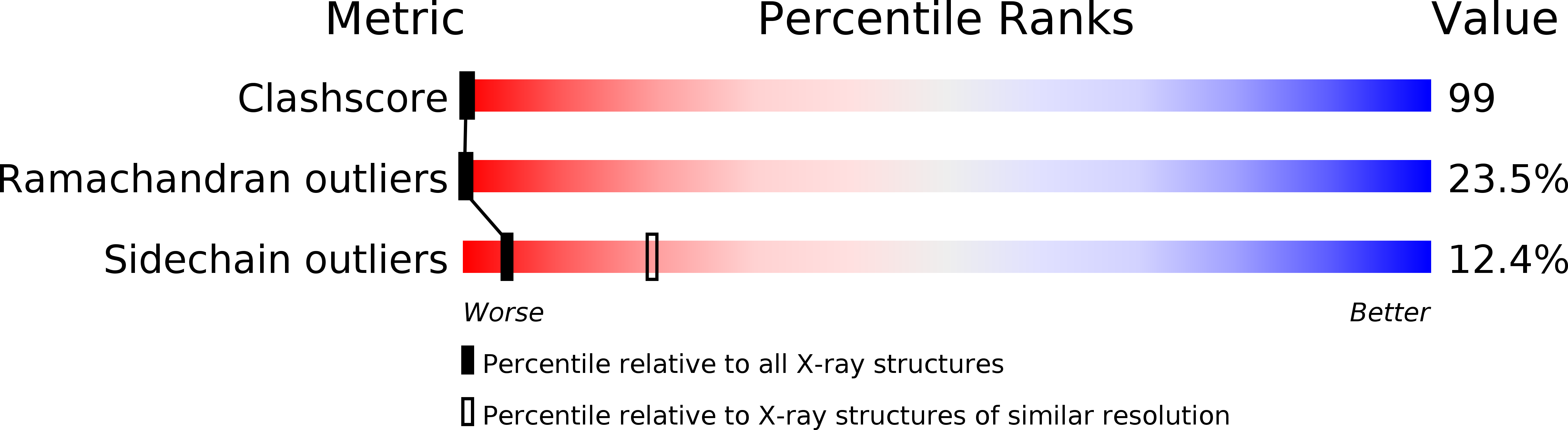
Deposition Date
2000-12-18
Release Date
2001-02-07
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1HQM
Keywords:
Title:
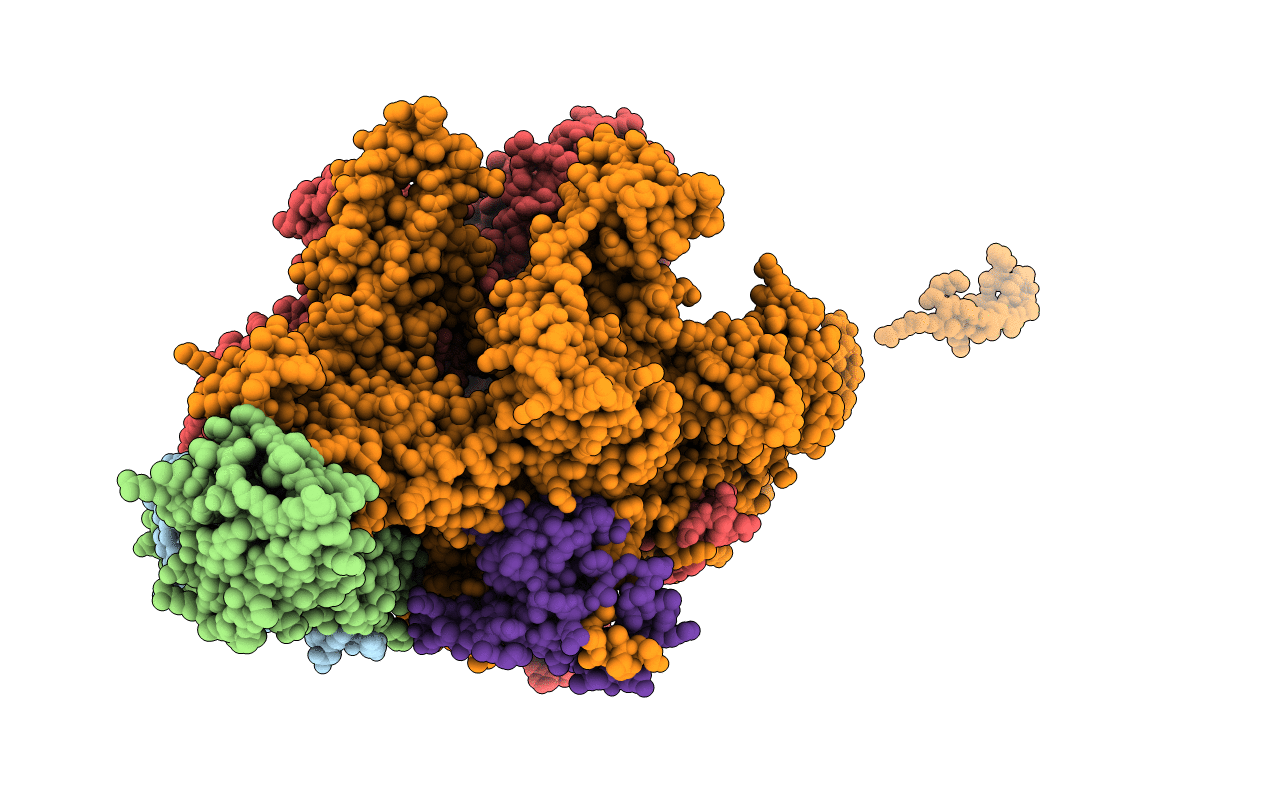
CRYSTAL STRUCTURE OF THERMUS AQUATICUS CORE RNA POLYMERASE-INCLUDES COMPLETE STRUCTURE WITH SIDE-CHAINS (EXCEPT FOR DISORDERED REGIONS)-FURTHER REFINED FROM ORIGINAL DEPOSITION-CONTAINS ADDITIONAL SEQUENCE INFORMATION
Biological Source:
Source Organism(s):
Thermus aquaticus (Taxon ID: 271)
Method Details:
Experimental Method:
Resolution:
3.30 Å
R-Value Free:
0.36
R-Value Work:
0.30
R-Value Observed:
0.30
Space Group:
P 41 21 2