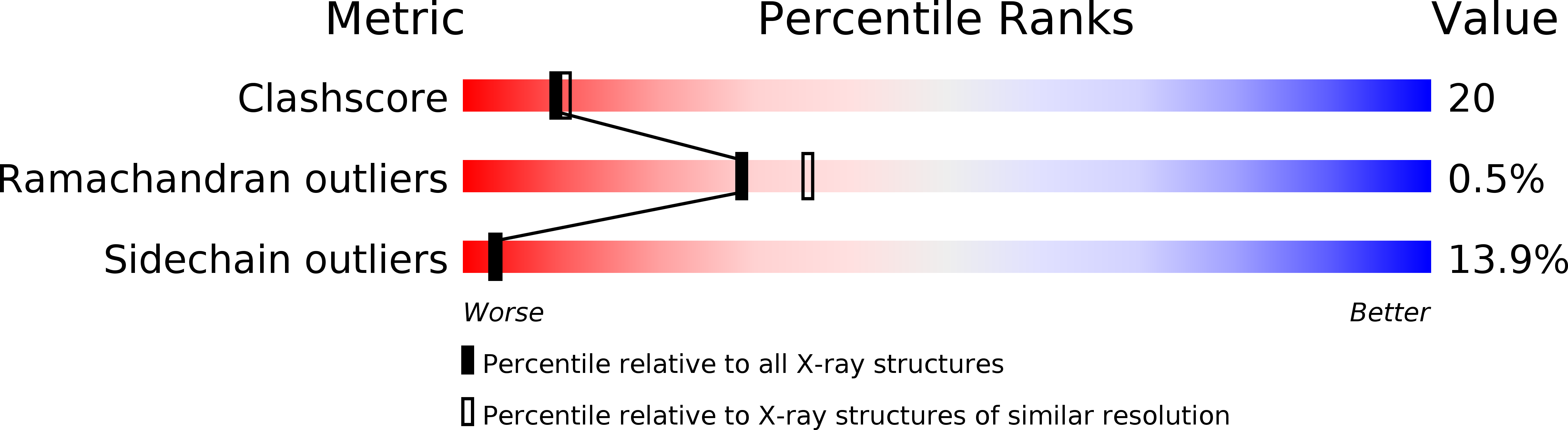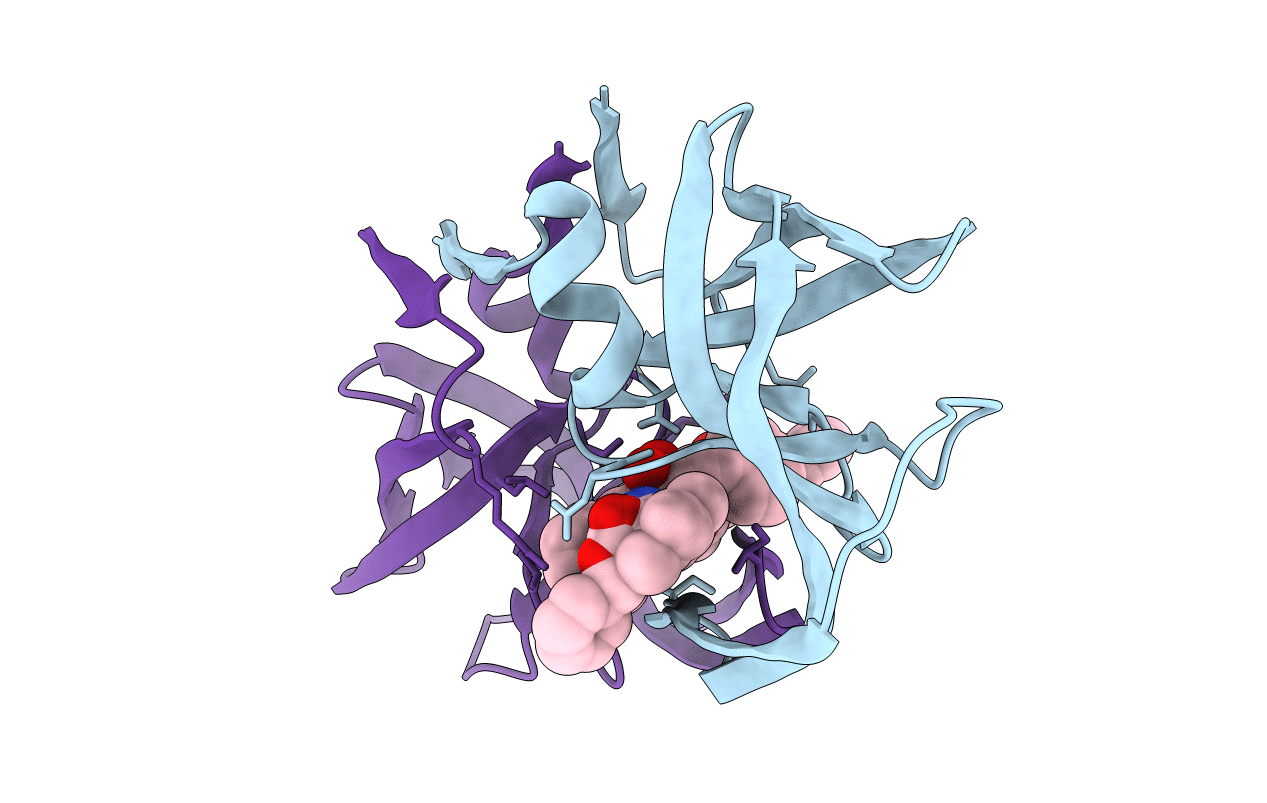
Deposition Date
1994-05-24
Release Date
1994-08-31
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1HPS
Keywords:
Title:
RATIONAL DESIGN, SYNTHESIS AND CRYSTALLOGRAPHIC ANALYSIS OF A HYDROXYETHYLENE-BASED HIV-1 PROTEASE INHIBITOR CONTAINING A HETEROCYCLIC P1'-P2' AMIDE BOND ISOSTERE
Biological Source:
Source Organism(s):
Human immunodeficiency virus 1 (Taxon ID: 11676)
Expression System(s):
Method Details: