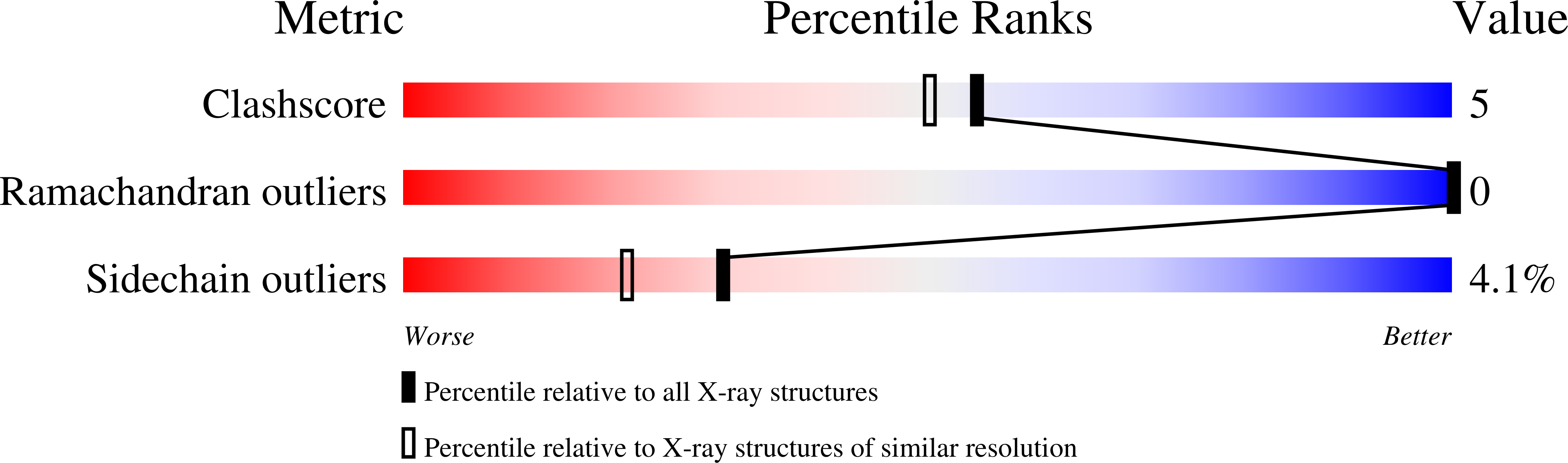
Deposition Date
2000-12-04
Release Date
2001-02-28
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1HLW
Keywords:
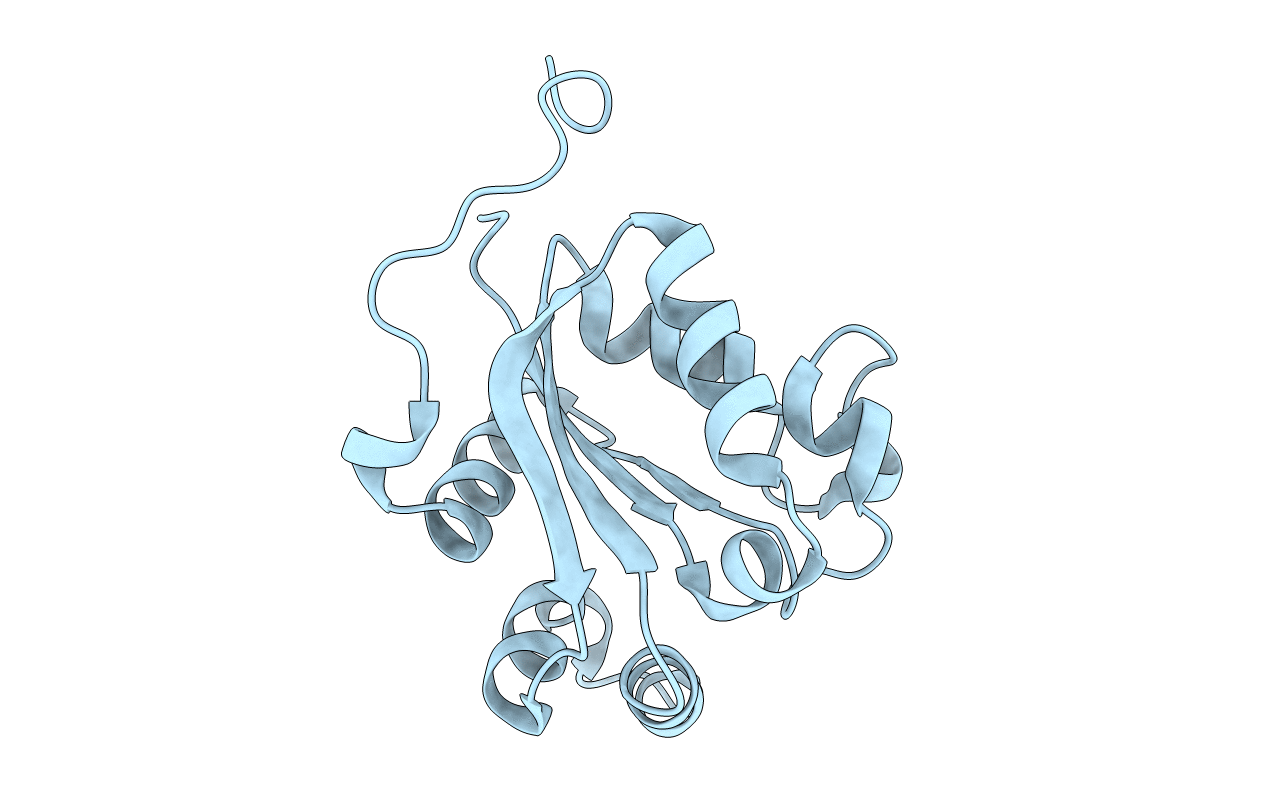
Title:
STRUCTURE OF THE H122A MUTANT OF THE NUCLEOSIDE DIPHOSPHATE KINASE
Biological Source:
Source Organism:
Dictyostelium discoideum (Taxon ID: 44689)
Host Organism:
Method Details:
Experimental Method:
Resolution:
1.90 Å
R-Value Free:
0.20
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 63 2 2