Deposition Date
2003-02-27
Release Date
2003-07-25
Last Version Date
2024-10-23
Entry Detail
PDB ID:
1HJQ
Keywords:
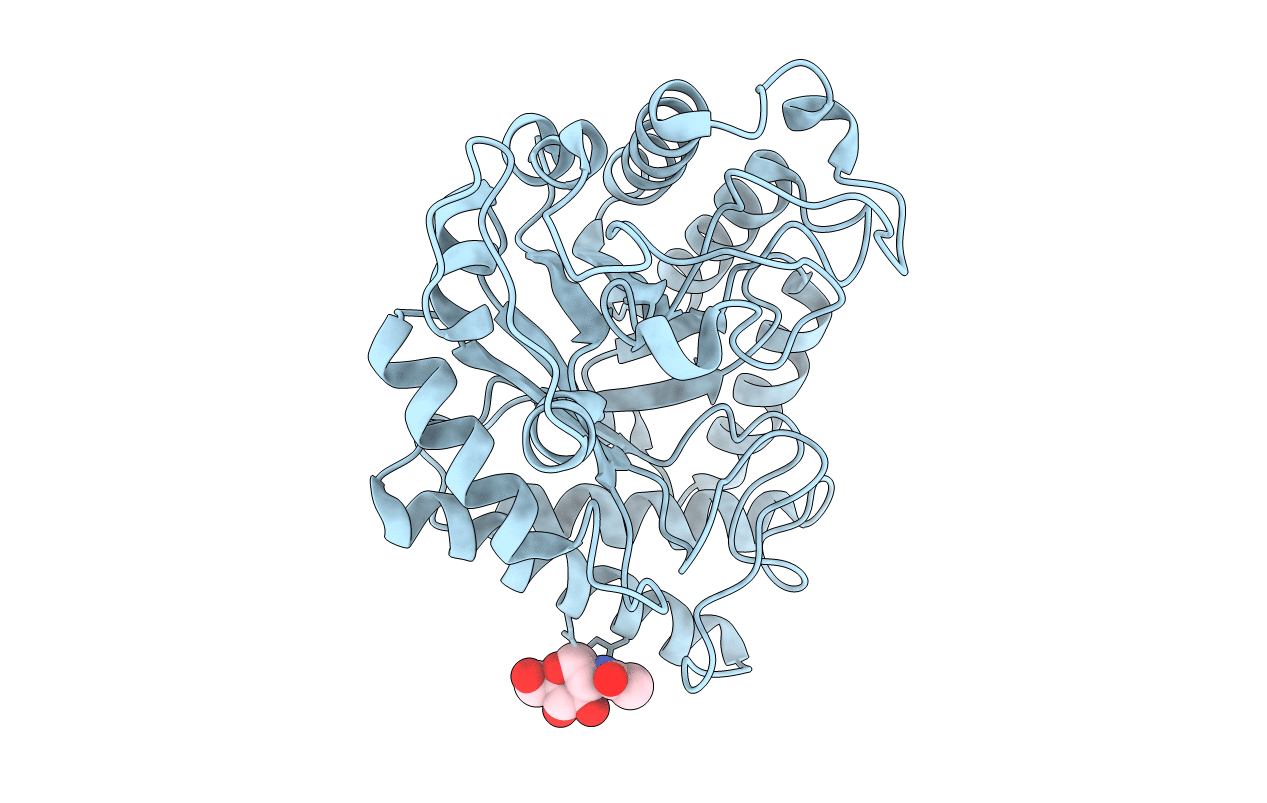
Title:
Structure of two fungal beta-1,4-galactanases: searching for the basis for temperature and pH optimum.
Biological Source:
Source Organism:
HUMICOLA INSOLENS (Taxon ID: 34413)
Host Organism:
Method Details:
Experimental Method:
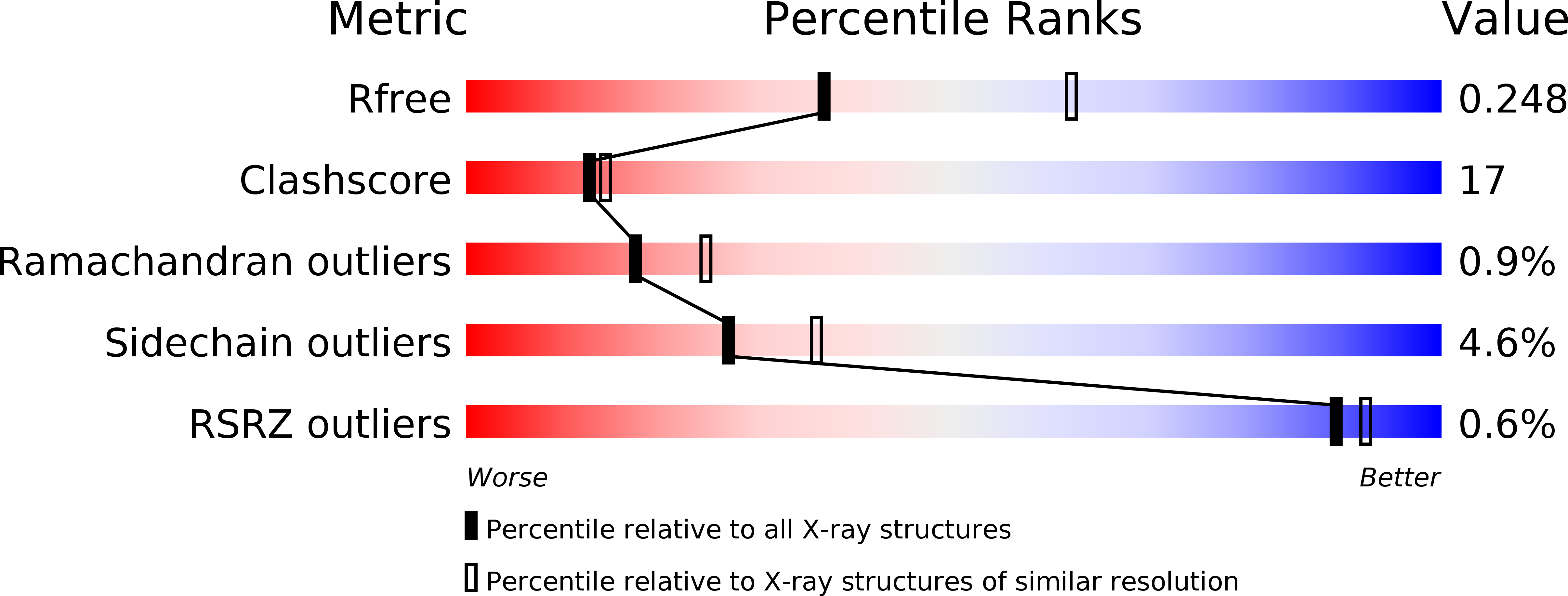
Resolution:
2.55 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 21