Deposition Date
2001-01-10
Release Date
2002-01-04
Last Version Date
2025-10-01
Entry Detail
PDB ID:
1HJ9
Keywords:
Title:
Atomic resolution structures of trypsin provide insight into structural radiation damage
Biological Source:
Source Organism:
BOS TAURUS (Taxon ID: 9913)
Method Details:
Experimental Method:
Resolution:
0.95 Å
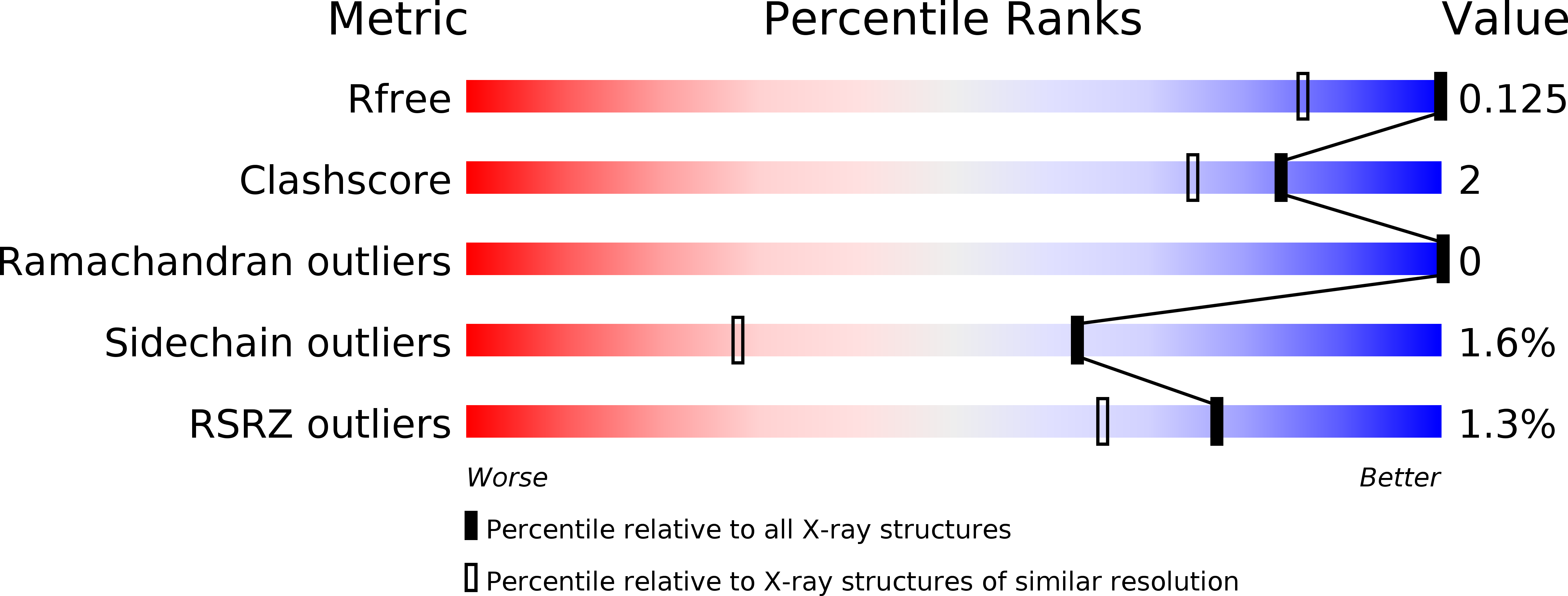
R-Value Free:
0.13
R-Value Observed:
0.11
Space Group:
P 21 21 21