Deposition Date
2001-01-05
Release Date
2001-11-30
Last Version Date
2023-12-13
Entry Detail
PDB ID:
1HIX
Keywords:
Title:
CRYSTALLOGRAPHIC ANALYSES OF FAMILY 11 ENDO-BETA-1,4-XYLANASE XYL1 FROM STREPTOMYCES SP. S38
Biological Source:
Source Organism(s):
STREPTOMYCES SP. S38 (Taxon ID: 181109)
Expression System(s):
Method Details:
Experimental Method:
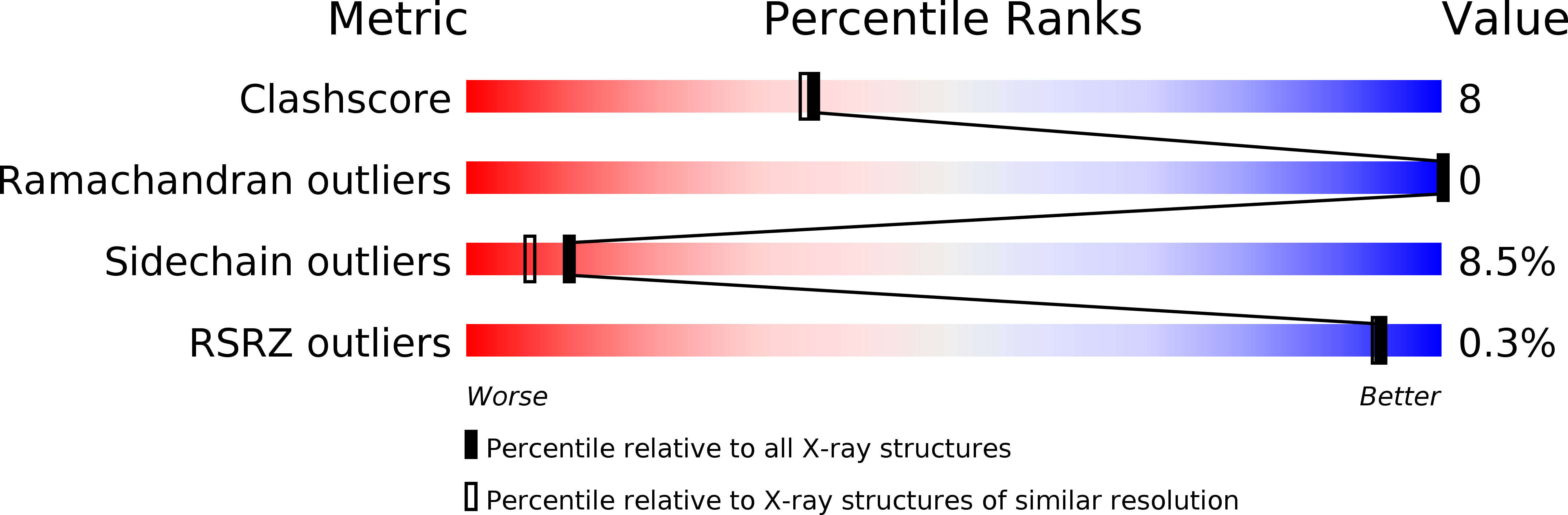
Resolution:
2.00 Å
R-Value Free:
0.30
R-Value Observed:
0.20
Space Group:
P 3 2 1