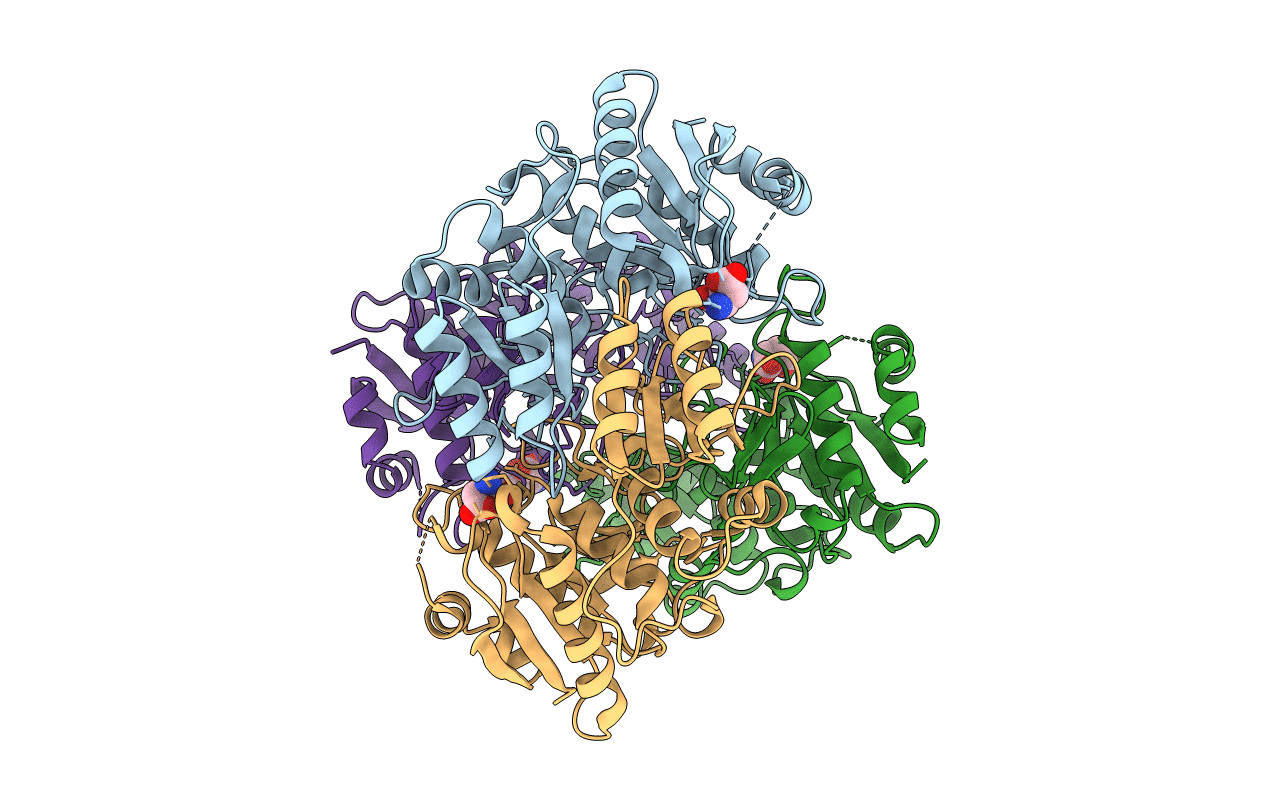
Deposition Date
2000-12-08
Release Date
2001-08-07
Last Version Date
2024-05-01
Entry Detail
PDB ID:
1HG1
Keywords:
Title:
X-ray structure of the complex between Erwinia chrysanthemi L-asparaginase and D-aspartate
Biological Source:
Source Organism(s):
ERWINIA CHRYSANTHEMI (Taxon ID: 556)
Method Details:
Experimental Method:
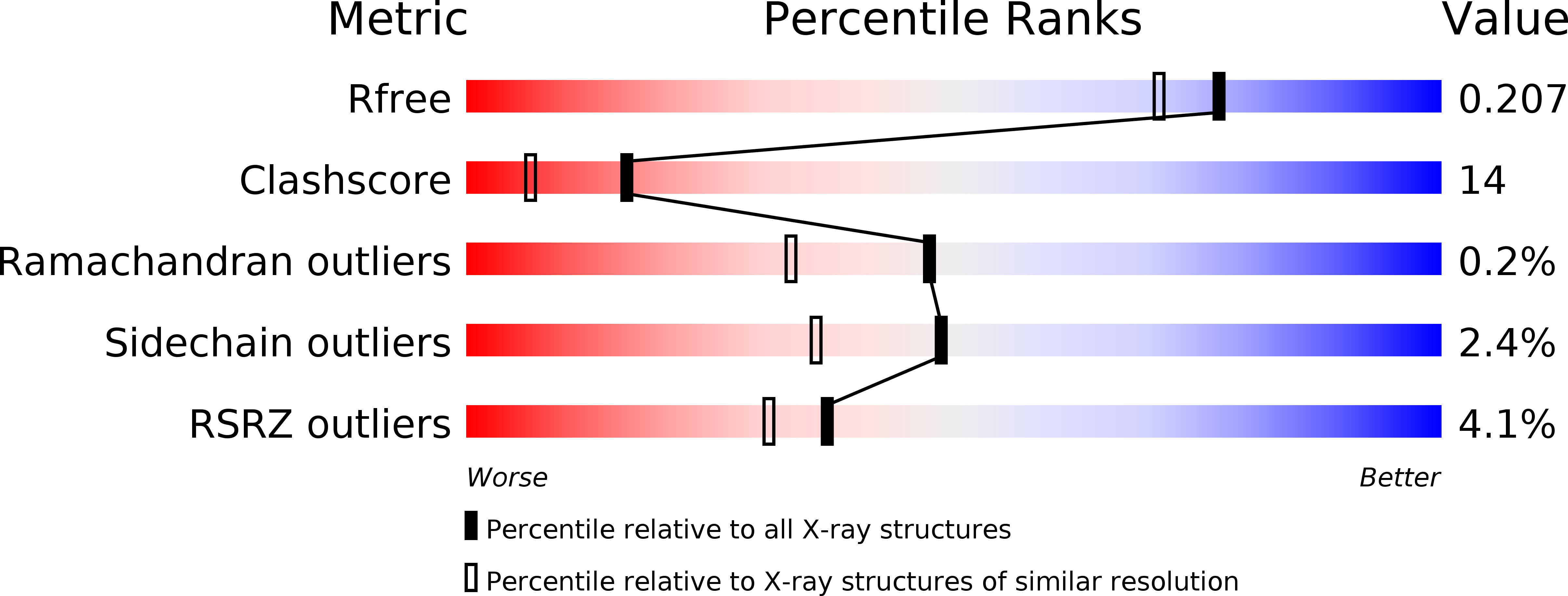
Resolution:
1.80 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 1 2 1