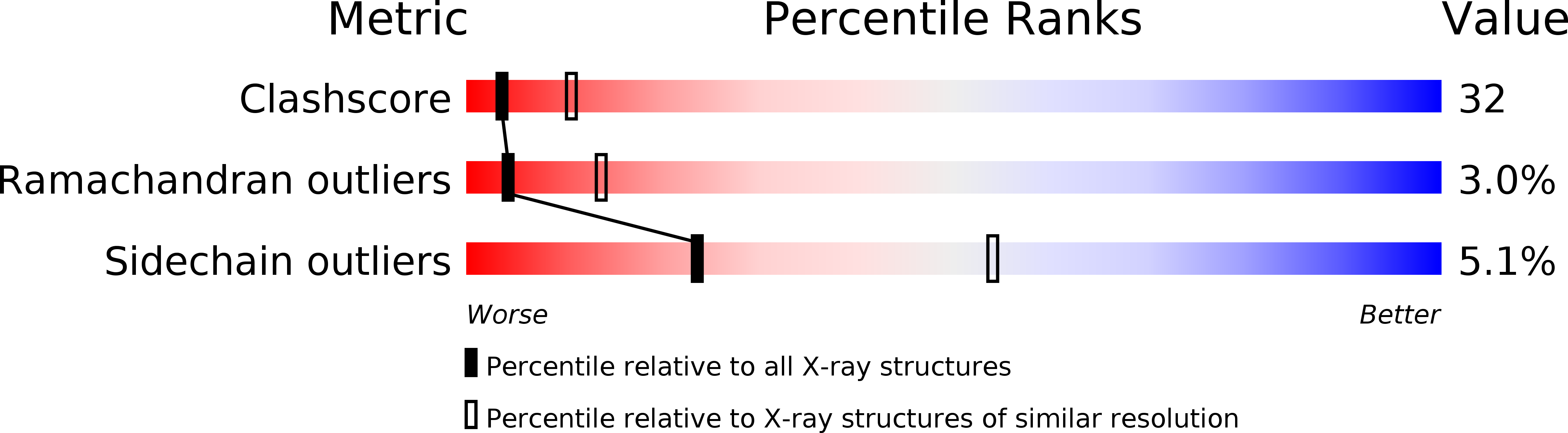Deposition Date
2001-03-28
Release Date
2002-04-03
Last Version Date
2024-11-20
Entry Detail
PDB ID:
1HA5
Keywords:
Title:
Structural features of a zinc-binding site in the superantigen streptococcal pyrogenic exotoxin A (SpeA1): implications for MHC class II recognition.
Biological Source:
Source Organism(s):
STREPTOCOCCUS PYOGENES (Taxon ID: 1314)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.82 Å
R-Value Free:
0.28
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 21 21 2