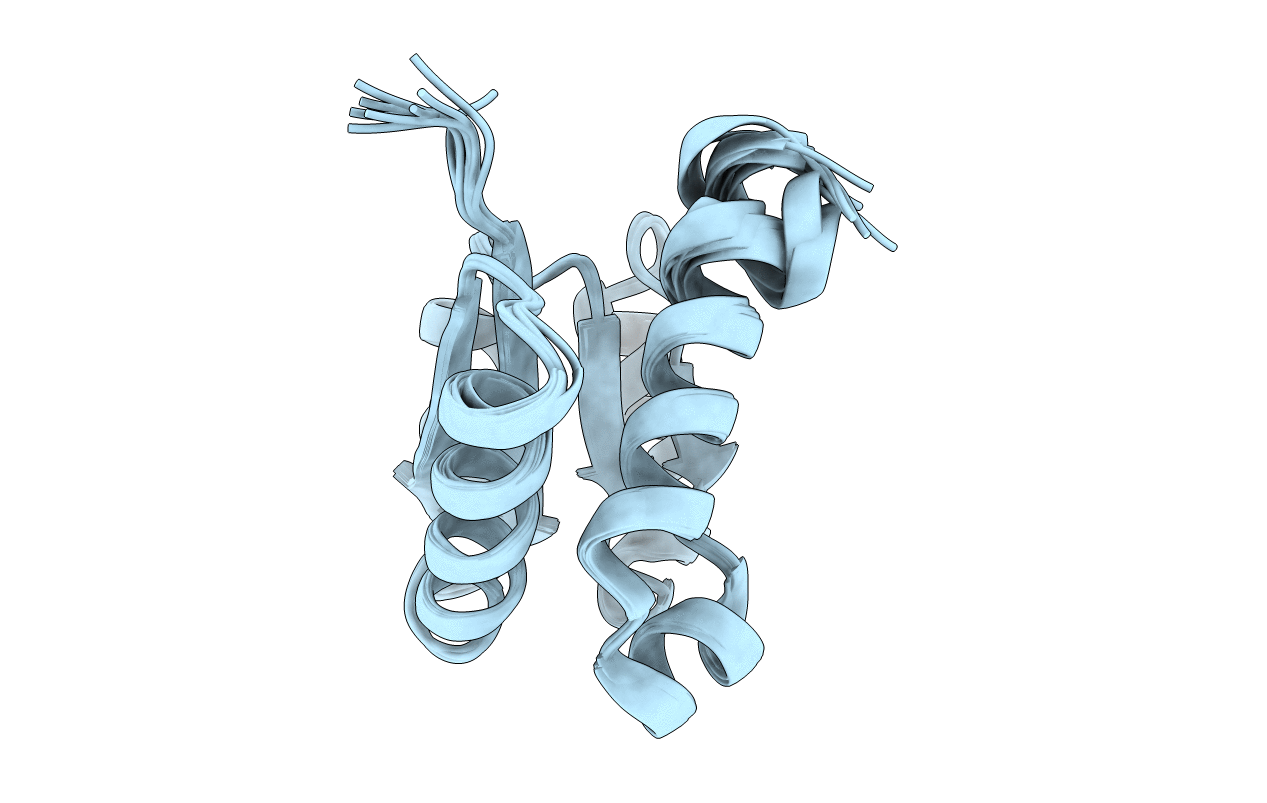
Deposition Date
2001-03-07
Release Date
2001-05-21
Last Version Date
2024-10-23
Entry Detail
PDB ID:
1H9C
Keywords:
Title:
NMR structure of cysteinyl-phosphorylated enzyme IIB of the N,N'-diacetylchitobiose specific phosphoenolpyruvate-dependent phosphotransferase system of Escherichia coli.
Biological Source:
Source Organism(s):
ESCHERICHIA COLI (Taxon ID: 316407)
Expression System(s):
Method Details:
Experimental Method:
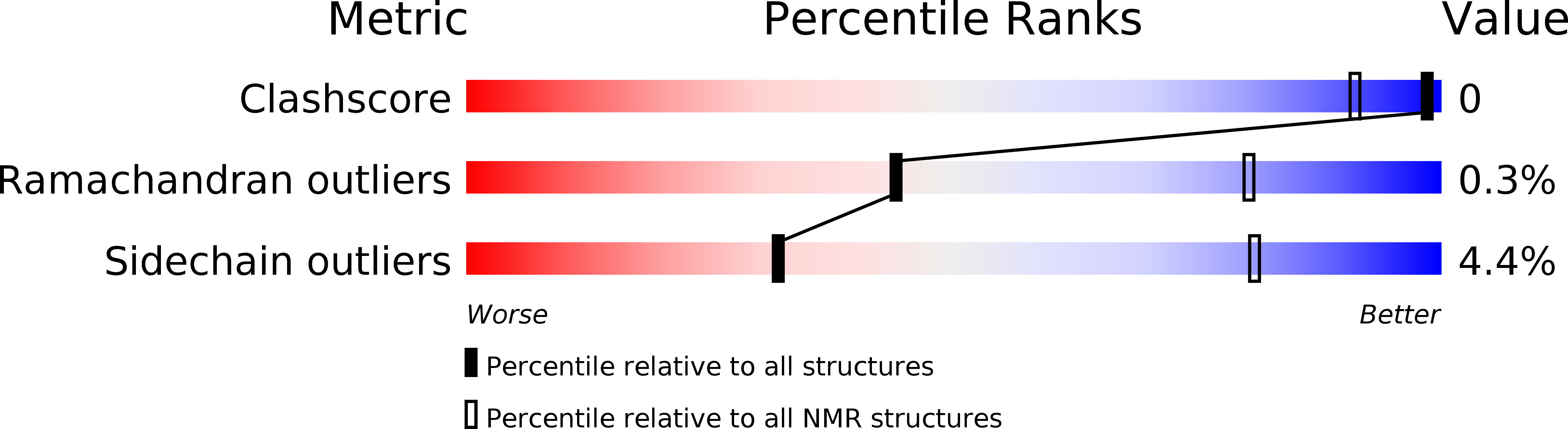
Conformers Calculated:
33
Conformers Submitted:
9
Selection Criteria:
TARGET-FUNCTION, R-FACTOR, NUMBER OF VIOLATIONS