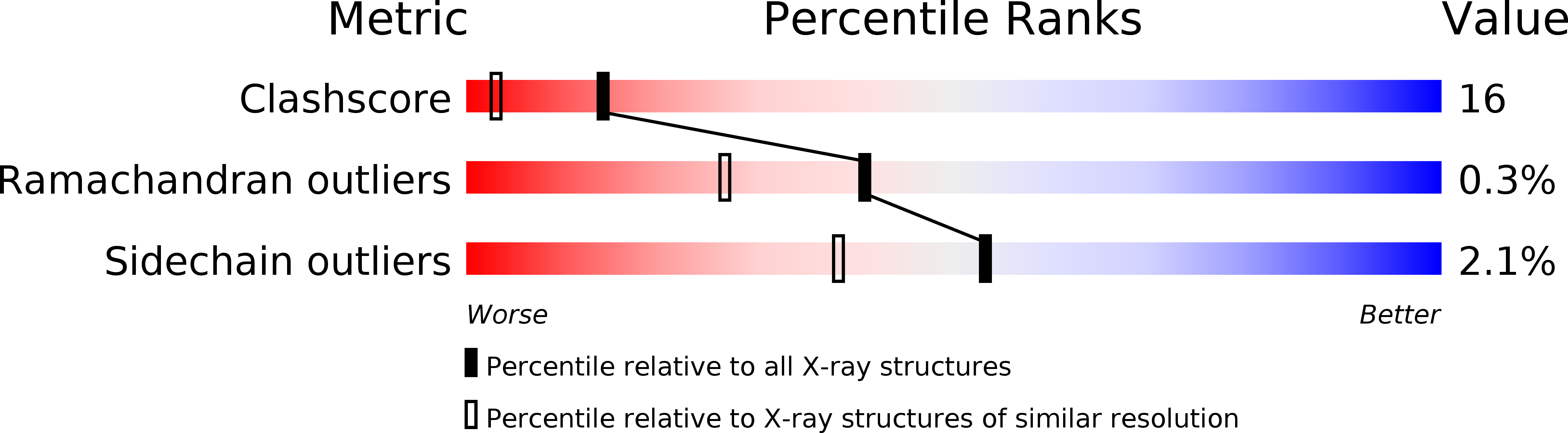Deposition Date
2001-06-08
Release Date
2001-09-05
Last Version Date
2023-12-13
Entry Detail
PDB ID:
1H69
Keywords:
Title:
CRYSTAL STRUCTURE OF HUMAN NAD[P]H-QUINONE OXIDOREDUCTASE CO WITH 2,3,5,6,TETRAMETHYL-P-BENZOQUINONE (DUROQUINONE) AT 2.5 ANGSTROM RESOLUTION
Biological Source:
Source Organism(s):
HOMO SAPIENS (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.86 Å
R-Value Free:
0.27
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 1