Deposition Date
2001-05-23
Release Date
2003-05-09
Last Version Date
2024-12-04
Entry Detail
PDB ID:
1H5O
Keywords:
Title:
Solution structure of Crotamine, a neurotoxin from Crotalus durissus terrificus
Biological Source:
Source Organism:
Crotalus durissus terrificus (Taxon ID: 8732)
Method Details:
Experimental Method:
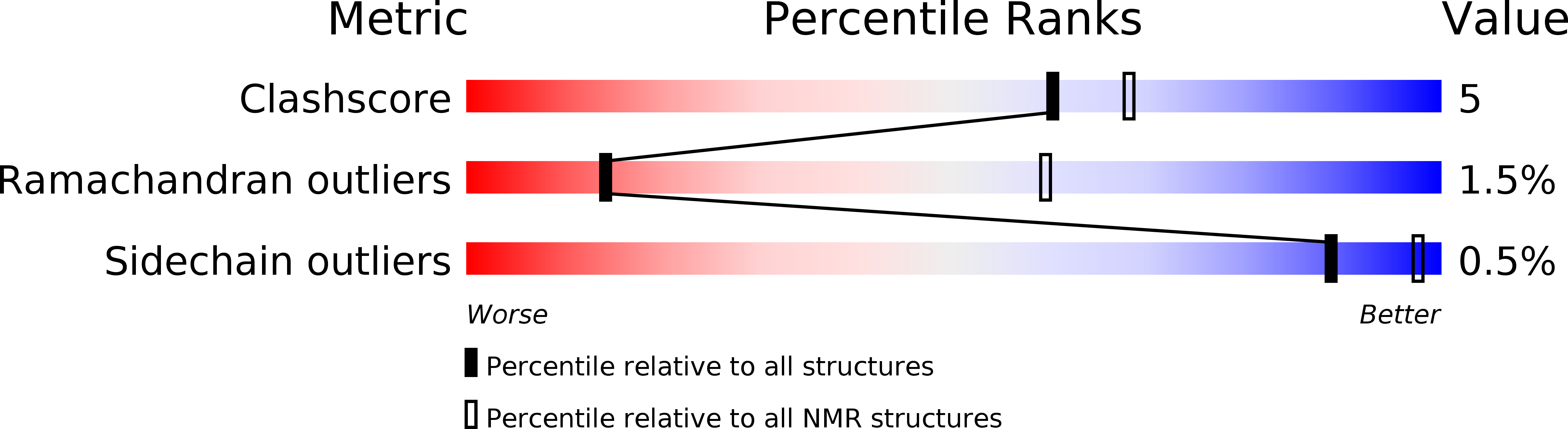
Conformers Calculated:
50
Conformers Submitted:
26
Selection Criteria:
LEAST RESTRAINT VIOLATION, LEAST RESIDUAL DEVIATIONS FROM IDEALIZED GEOMETRY