Deposition Date
2001-05-13
Release Date
2001-06-18
Last Version Date
2023-12-13
Entry Detail
PDB ID:
1H4Q
Keywords:
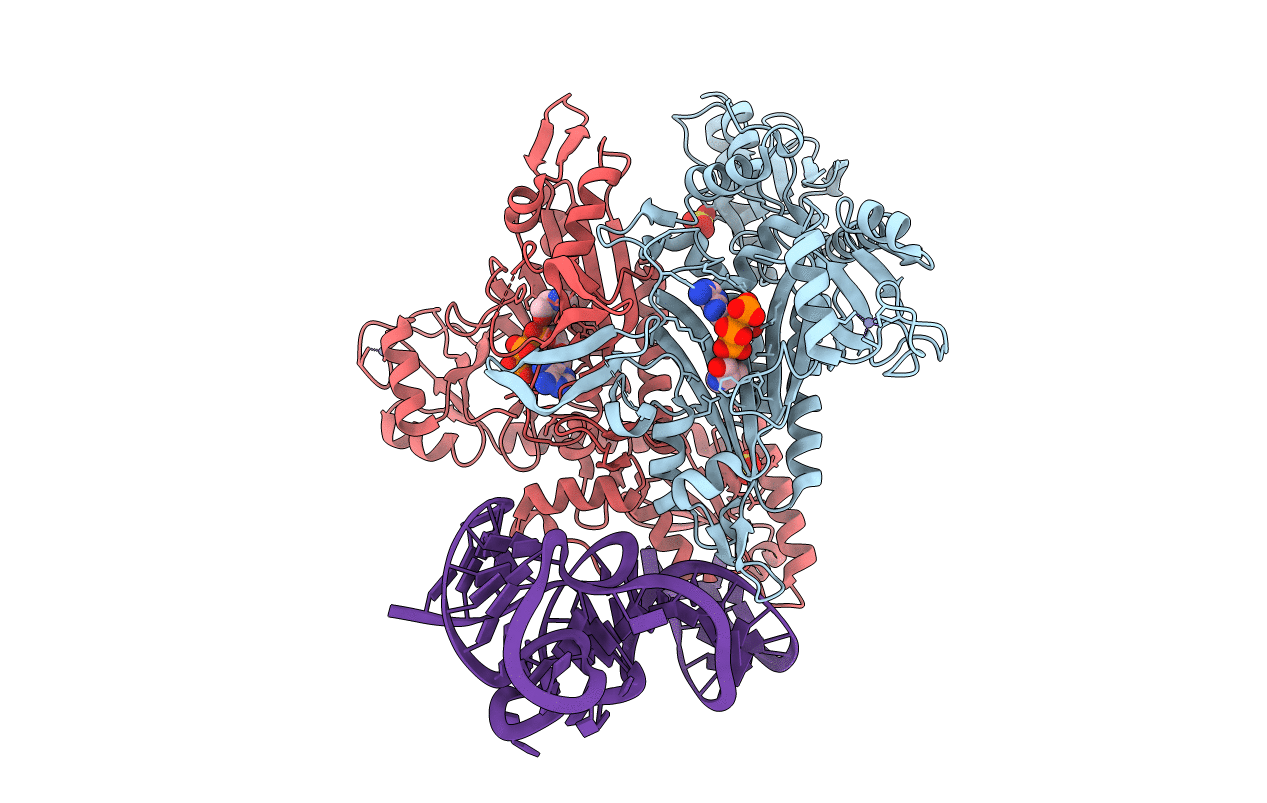
Title:
Prolyl-tRNA synthetase from Thermus thermophilus complexed with tRNApro(CGG), ATP and prolinol
Biological Source:
Source Organism(s):
THERMUS THERMOPHILUS (Taxon ID: 274)
Method Details:
Experimental Method:
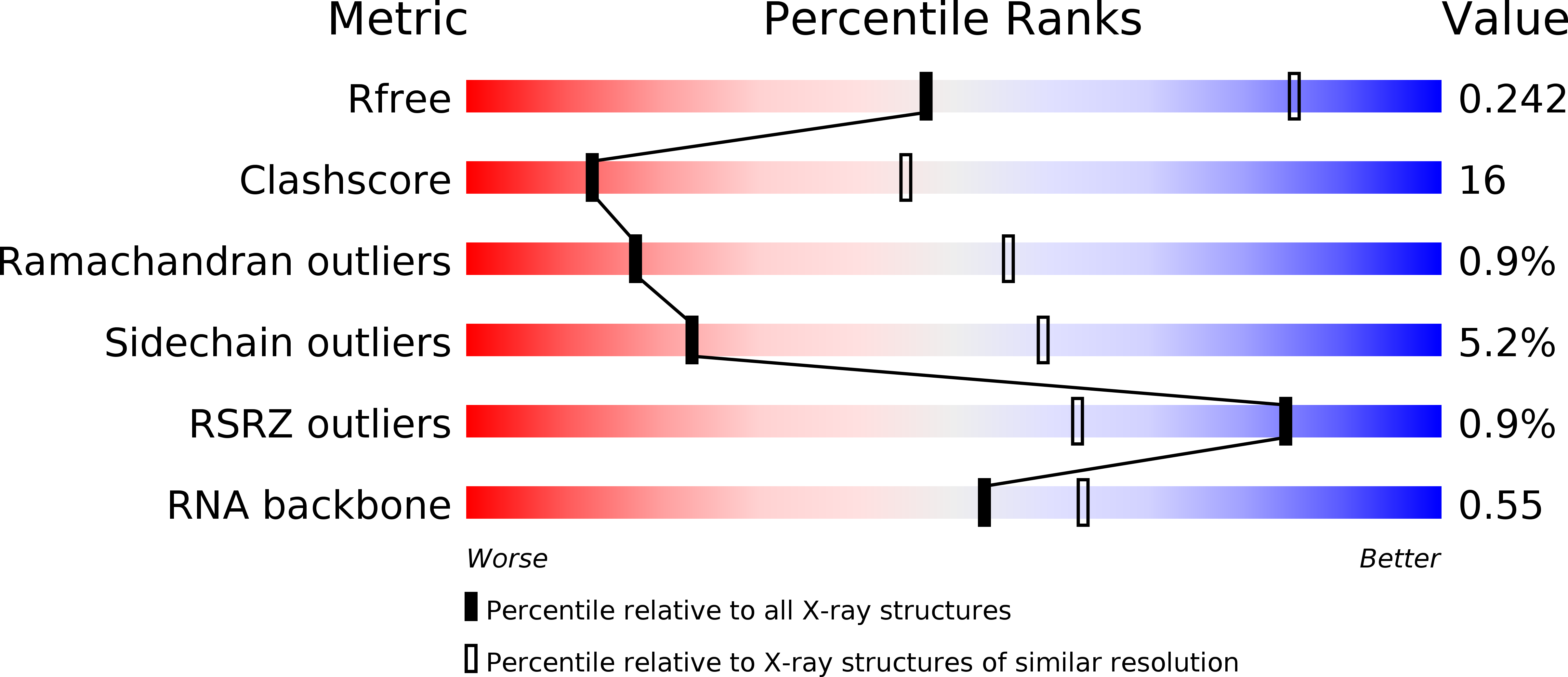
Resolution:
3.00 Å
R-Value Free:
0.25
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 43 21 2