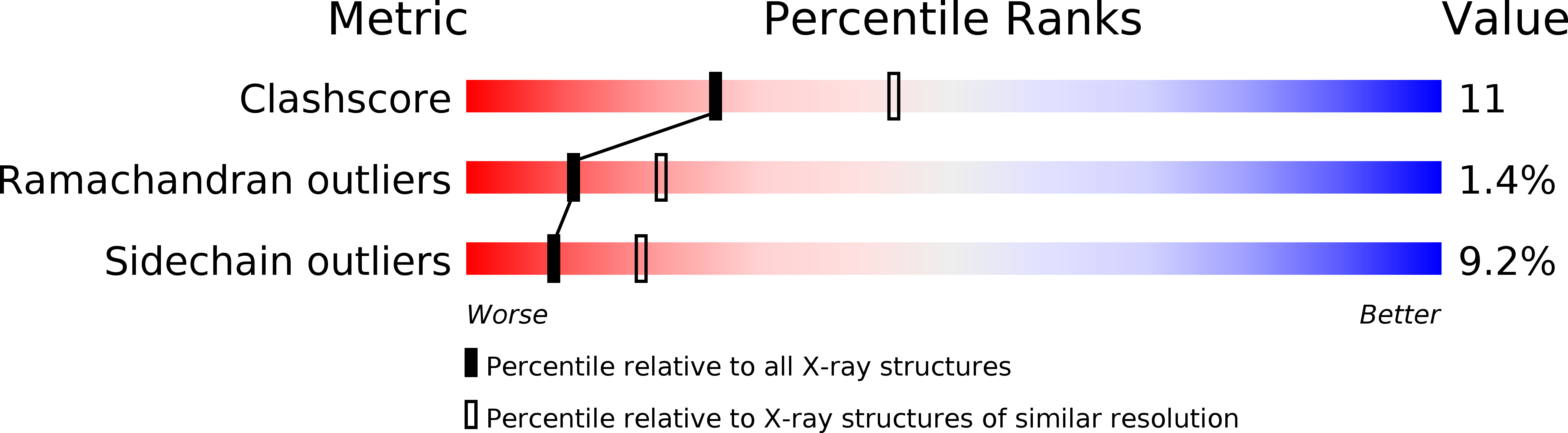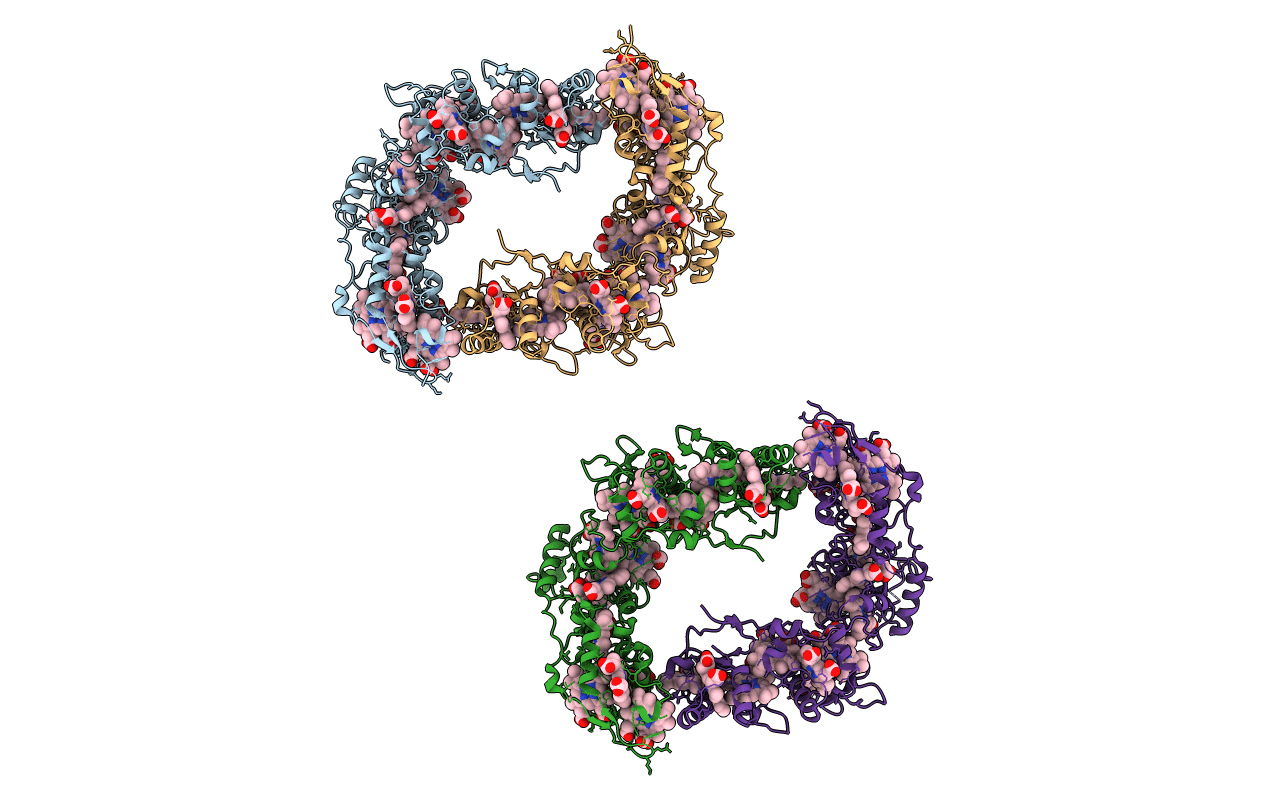
Deposition Date
2002-08-01
Release Date
2002-10-02
Last Version Date
2024-10-16
Entry Detail
PDB ID:
1H29
Keywords:
Title:
Sulfate respiration in Desulfovibrio vulgaris Hildenborough: Structure of the 16-heme Cytochrome c HmcA at 2.5 A resolution and a view of its role in transmembrane electron transfer
Biological Source:
Source Organism(s):
DESULFOVIBRIO VULGARIS (Taxon ID: 882)
Method Details:
Experimental Method:
Resolution:
2.51 Å
R-Value Free:
0.25
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 62