Deposition Date
2002-07-25
Release Date
2003-01-30
Last Version Date
2024-05-08
Entry Detail
PDB ID:
1H1Z
Keywords:
Title:
The structure of the cytosolic D-ribulose-5-phosphate 3-epimerase from rice complexed with sulfate and zinc
Biological Source:
Source Organism:
ORYZA SATIVA (Taxon ID: 4530)
Host Organism:
Method Details:
Experimental Method:
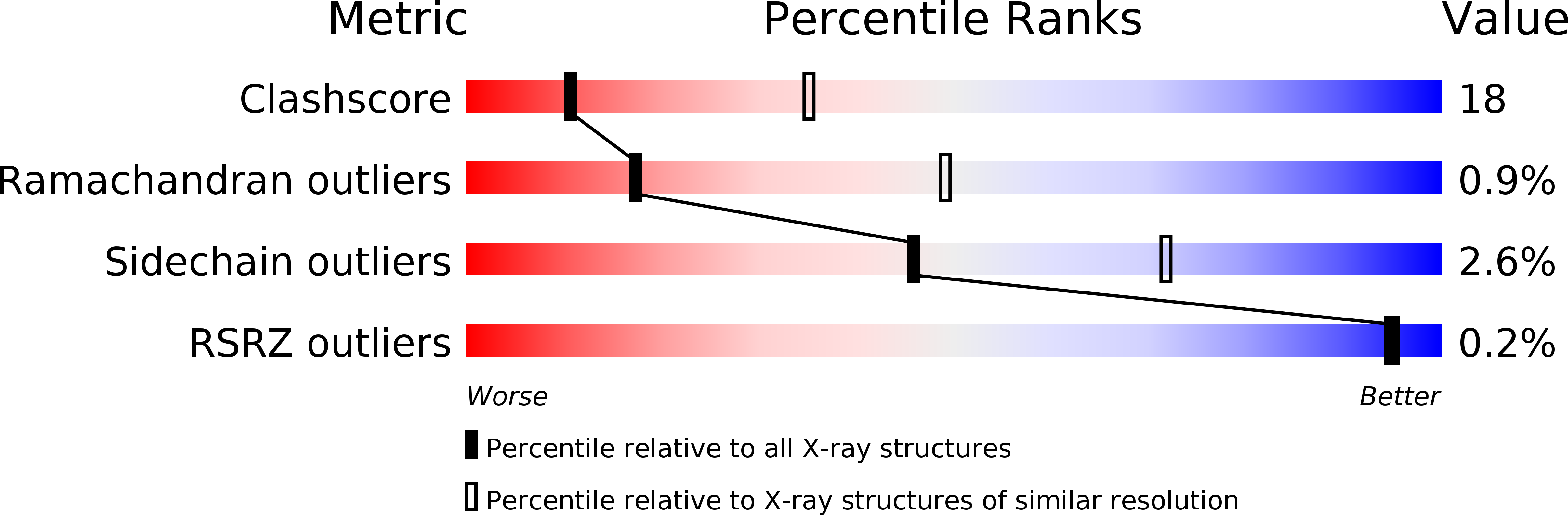
Resolution:
3.40 Å
R-Value Free:
0.25
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 1 21 1