Deposition Date
2002-05-17
Release Date
2002-06-21
Last Version Date
2024-05-01
Entry Detail
PDB ID:
1GZ9
Keywords:
Title:
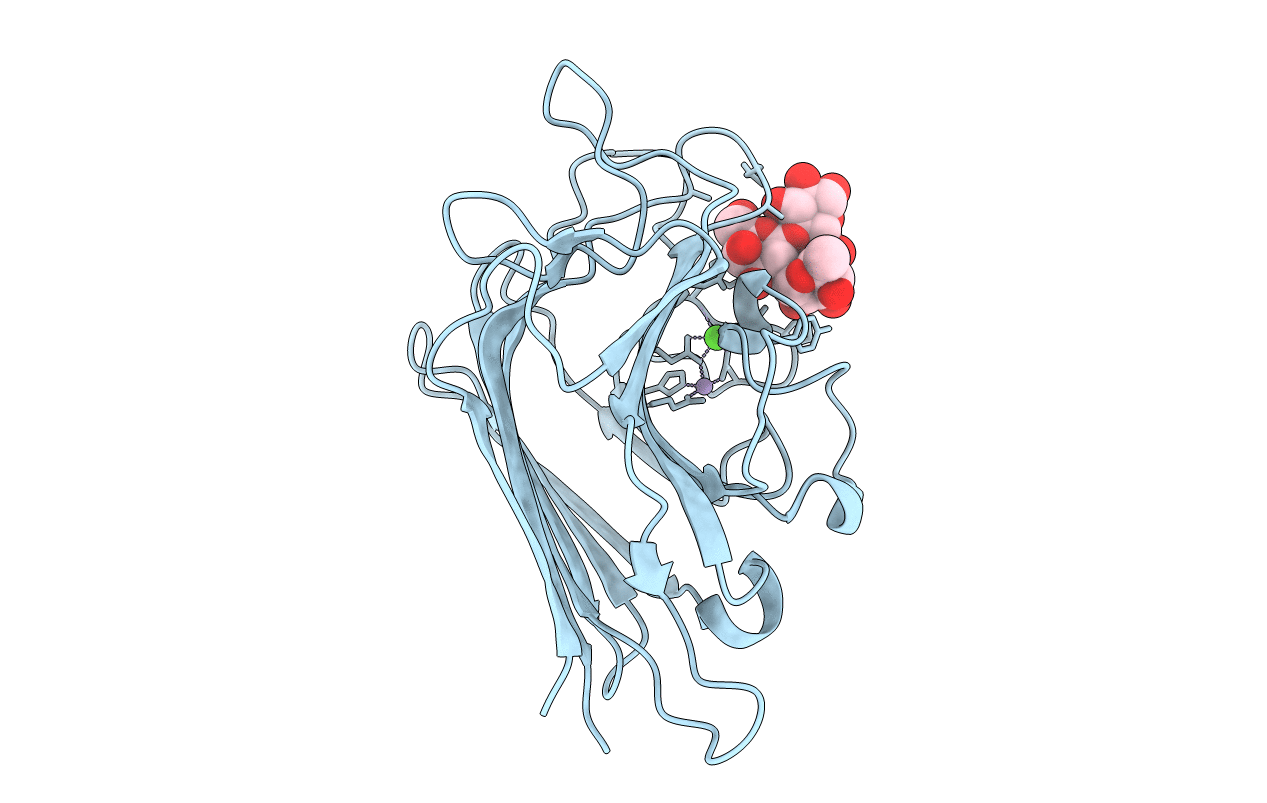
High-Resolution Crystal Structure of Erythrina cristagalli Lectin in Complex with 2'-alpha-L-Fucosyllactose
Biological Source:
Source Organism(s):
ERYTHRINA CRISTA-GALLI (Taxon ID: 49817)
Method Details:
Experimental Method:
Resolution:
1.70 Å
R-Value Free:
0.23
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 43 21 2


