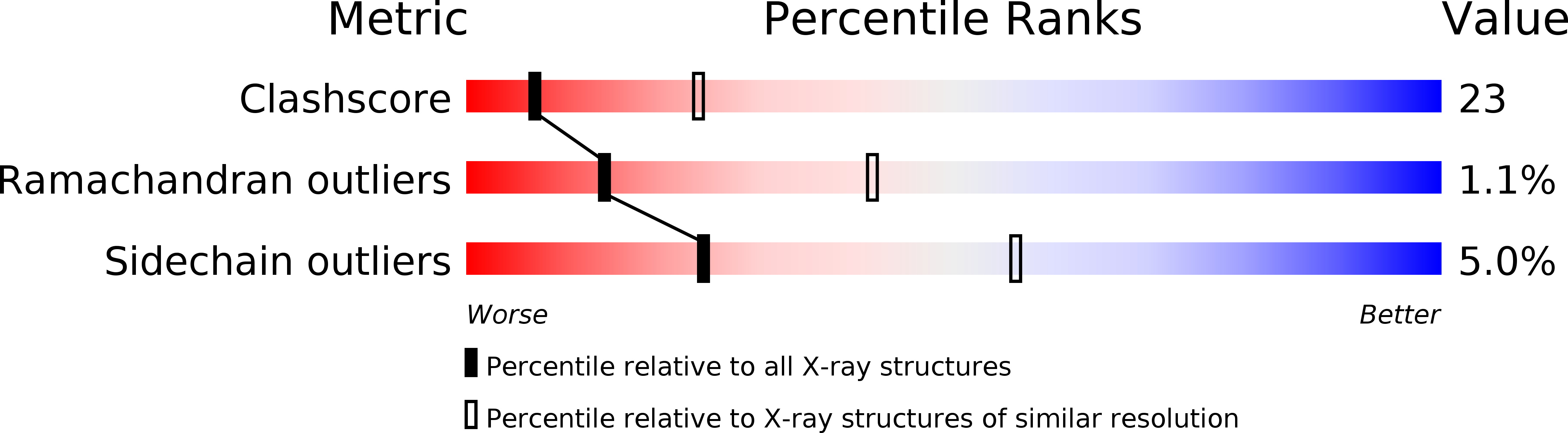
Deposition Date
2002-01-16
Release Date
2002-06-13
Last Version Date
2023-12-13
Entry Detail
PDB ID:
1GTL
Keywords:
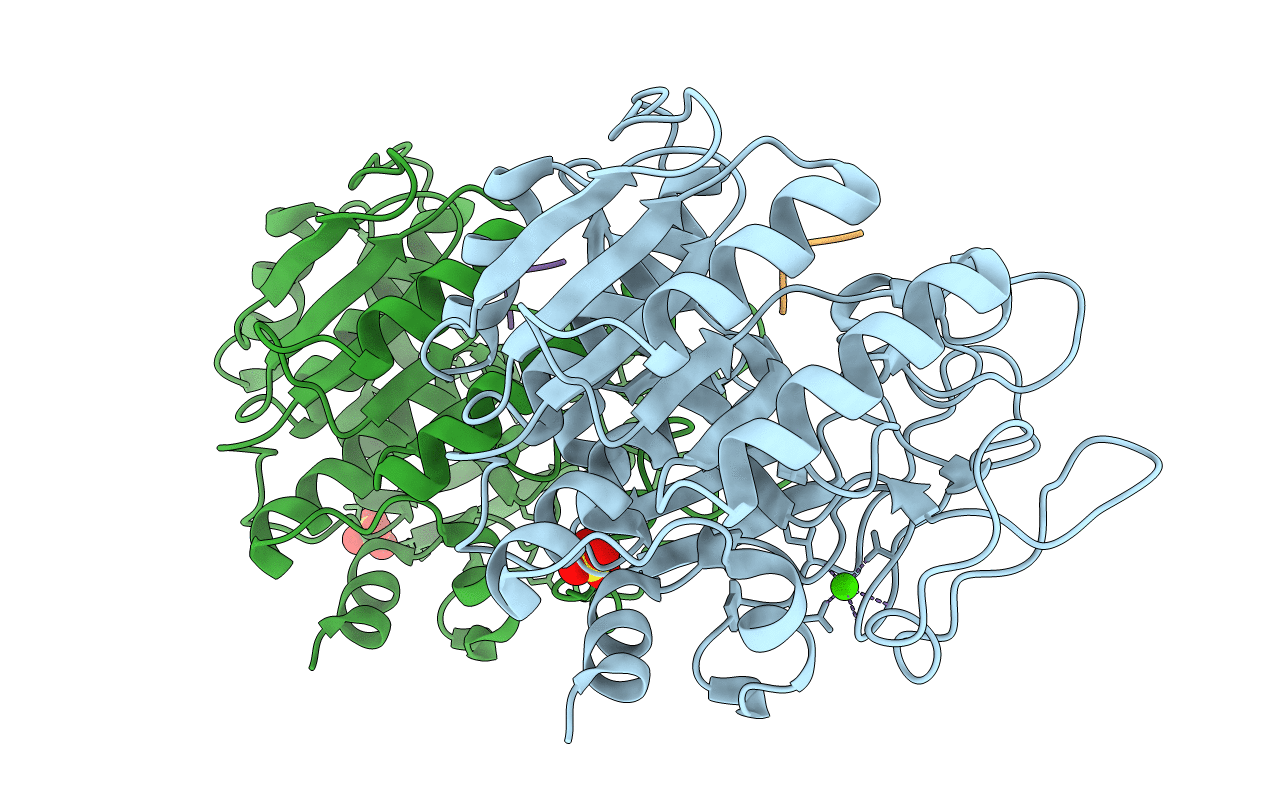
Title:
The thermostable serine-carboxyl type proteinase, kumamolisin (KSCP) - complex with Ac-Ile-Pro-Phe-cho
Biological Source:
Source Organism(s):
BACILLUS NOVOSP. MN-32 (Taxon ID: 198803)
SYNTHETIC CONSTRUCT (Taxon ID: 32630)
SYNTHETIC CONSTRUCT (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.80 Å
R-Value Free:
0.27
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 1 21 1