Deposition Date
2001-10-04
Release Date
2002-04-11
Last Version Date
2024-10-16
Entry Detail
PDB ID:
1GN9
Keywords:
Title:
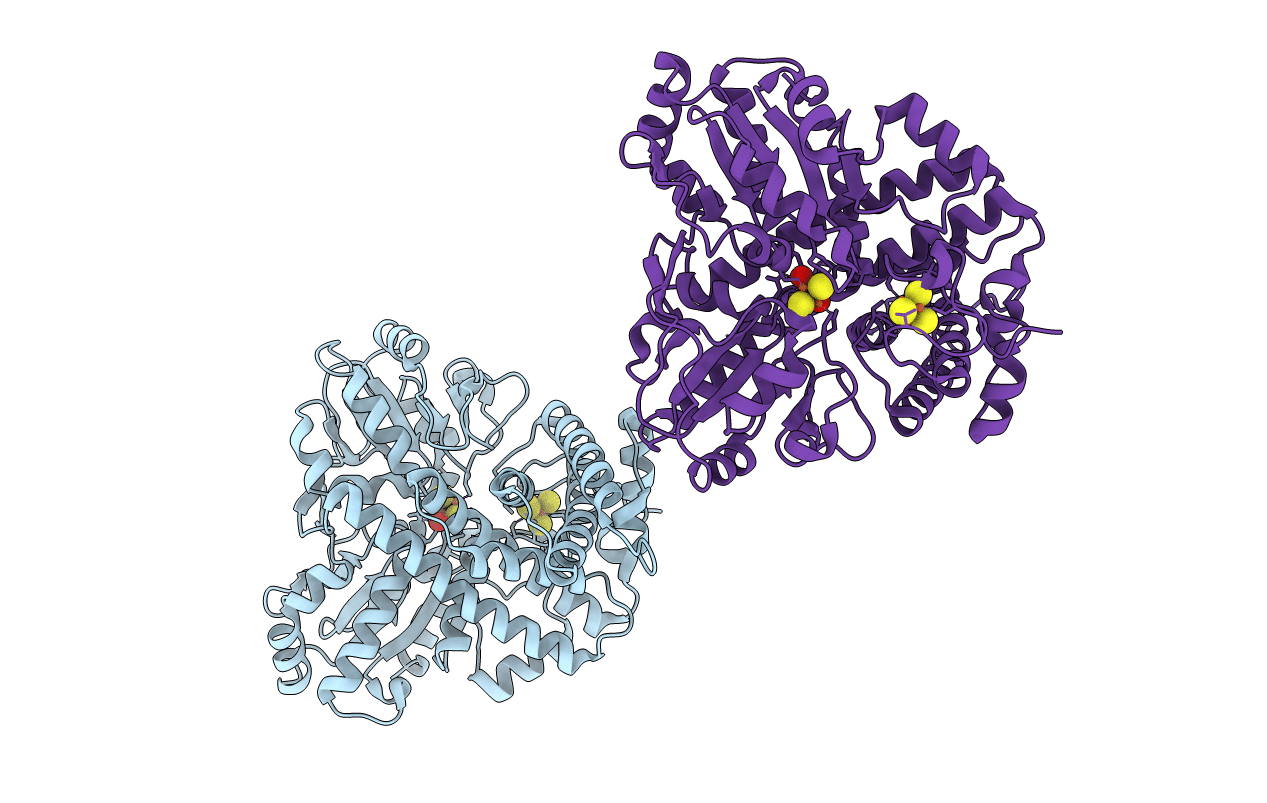
Hybrid Cluster Protein from Desulfovibrio desulfuricans ATCC 27774 X-ray structure at 2.6A resolution using synchrotron radiation at a wavelength of 1.722A
Biological Source:
Source Organism:
DESULFOVIBRIO DESULFURICANS (Taxon ID: 876)
Method Details:
Experimental Method:
Resolution:
2.60 Å
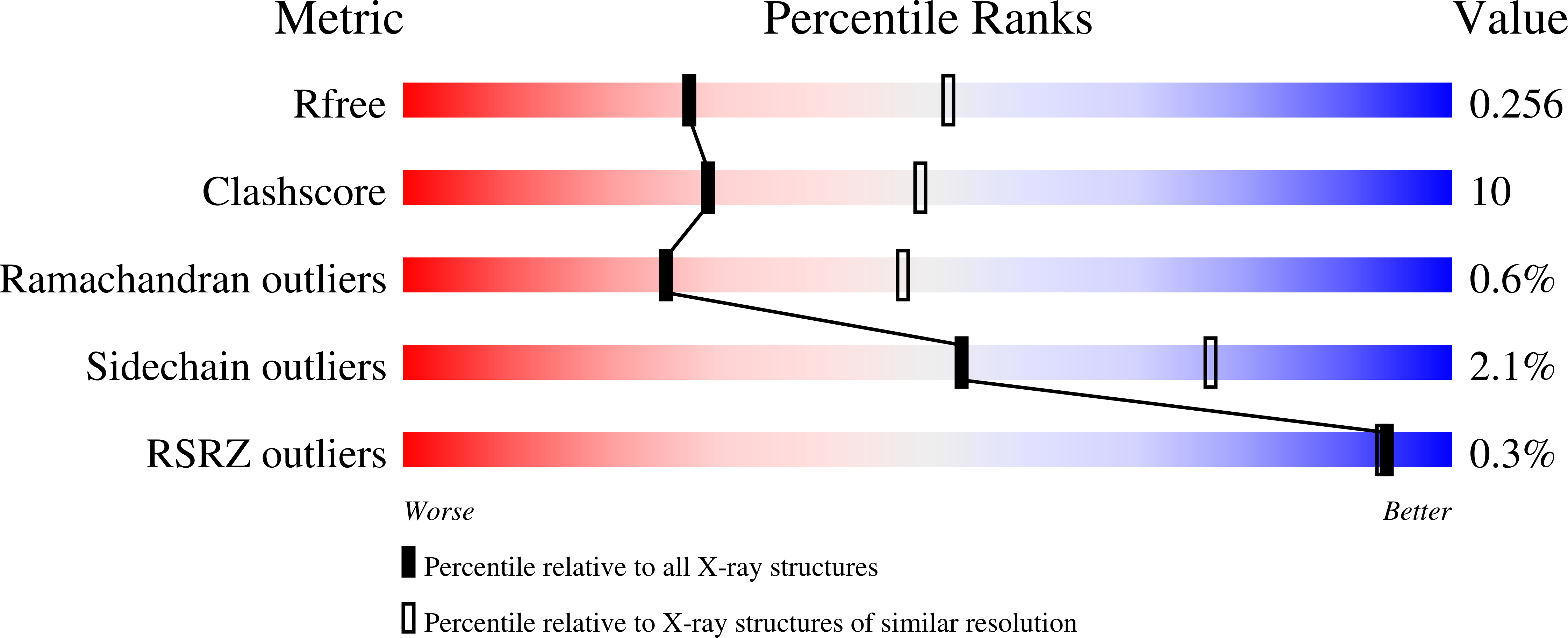
R-Value Free:
0.26
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 1