Deposition Date
2001-08-22
Release Date
2001-11-28
Last Version Date
2024-10-23
Entry Detail
PDB ID:
1GL0
Keywords:
Title:
structure of the complex between bovine alpha-chymotrypsin and PMP-D2v, an inhibitor from the insect Locusta migratoria
Biological Source:
Source Organism(s):
LOCUSTA MIGRATORIA (Taxon ID: 7004)
BOS TAURUS (Taxon ID: 9913)
BOS TAURUS (Taxon ID: 9913)
Method Details:
Experimental Method:
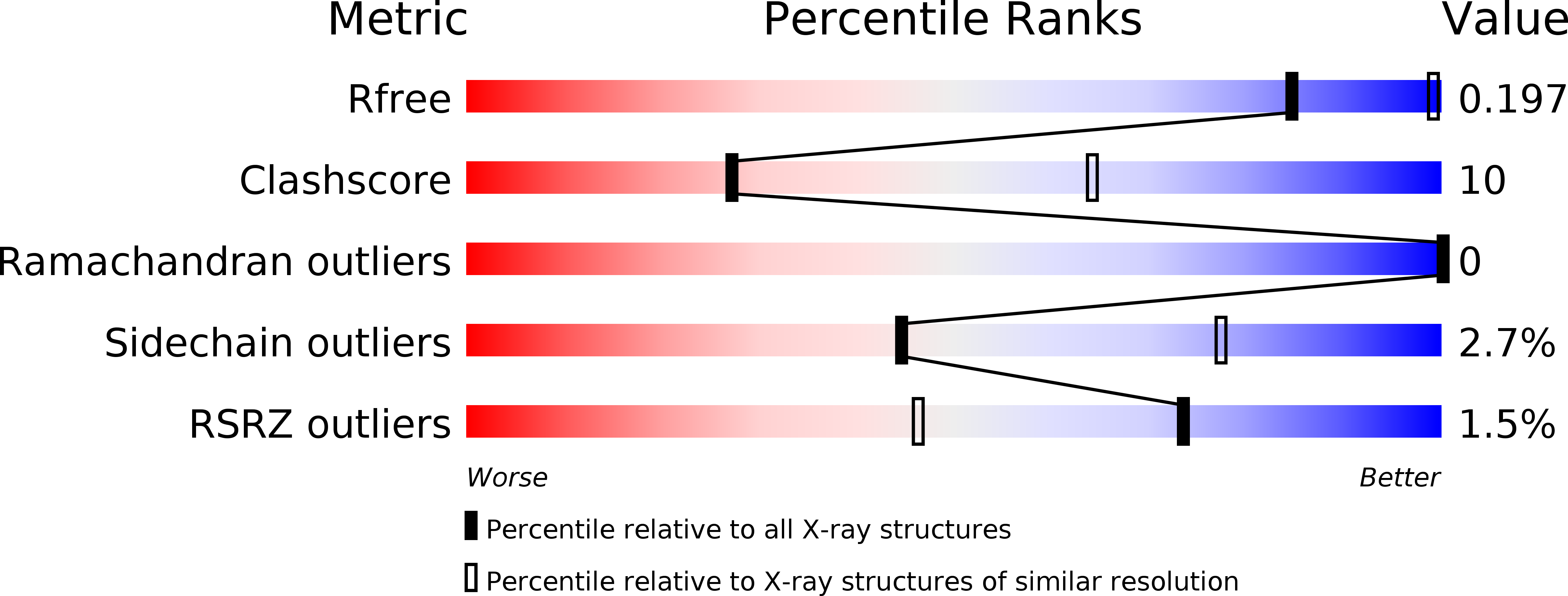
Resolution:
3.00 Å
R-Value Free:
0.19
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 65 2 2