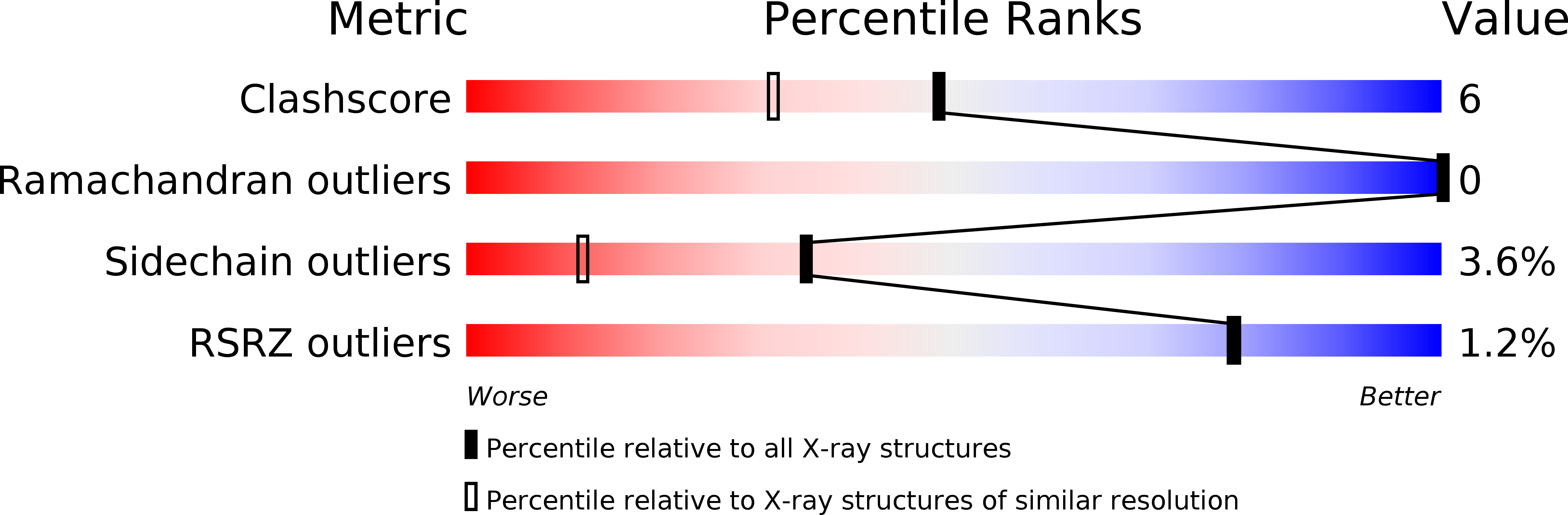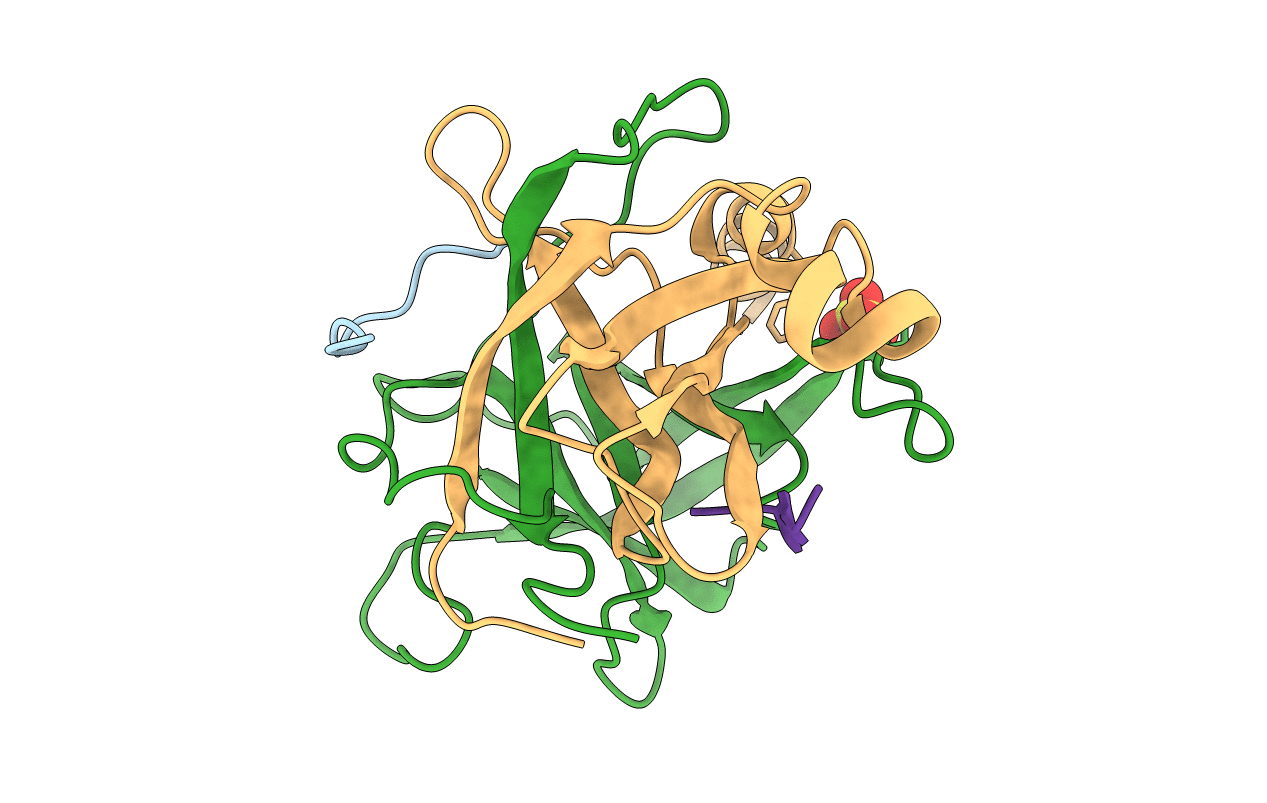
Deposition Date
1990-09-04
Release Date
1991-10-15
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1GCT
Keywords:
Title:
IS GAMMA-CHYMOTRYPSIN A TETRAPEPTIDE ACYL-ENZYME ADDUCT OF GAMMA-CHYMOTRYPSIN?
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Method Details: