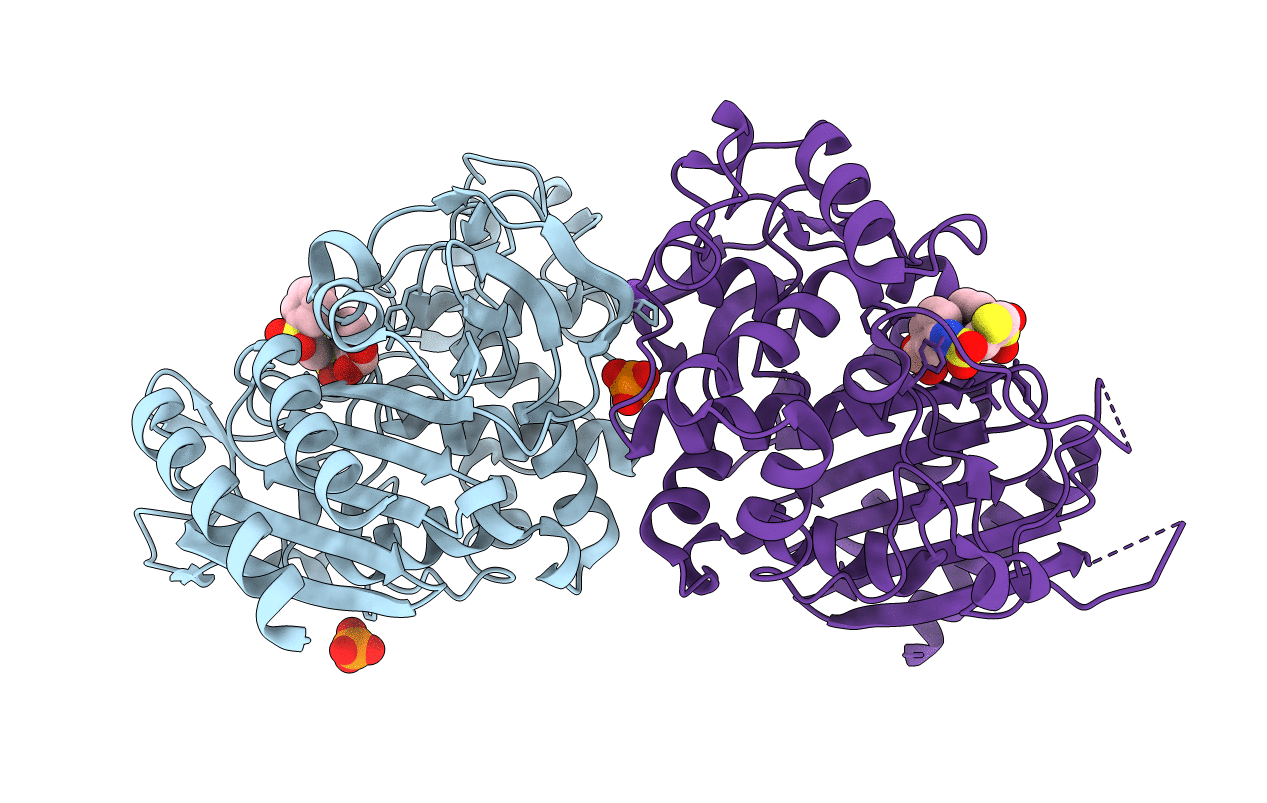
Deposition Date
2000-11-29
Release Date
2001-07-25
Last Version Date
2024-10-09
Entry Detail
PDB ID:
1GA9
Keywords:
Title:
CRYSTAL STRUCTURE OF AMPC BETA-LACTAMASE FROM E. COLI COMPLEXED WITH NON-BETA-LACTAMASE INHIBITOR (2, 3-(4-BENZENESULFONYL-THIOPHENE-2-SULFONYLAMINO)-PHENYLBORONIC ACID)
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 562)
Expression System(s):
Method Details:
Experimental Method:
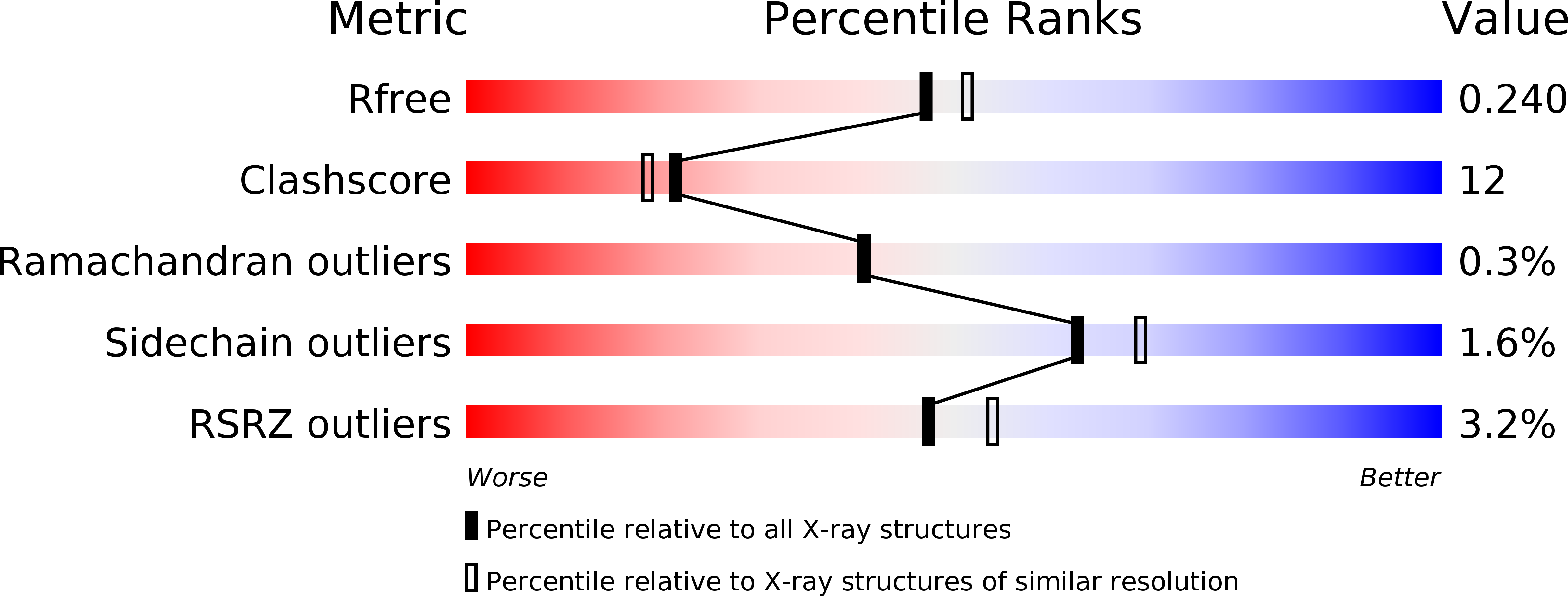
Resolution:
2.10 Å
R-Value Free:
0.24
R-Value Work:
0.20
Space Group:
C 1 2 1