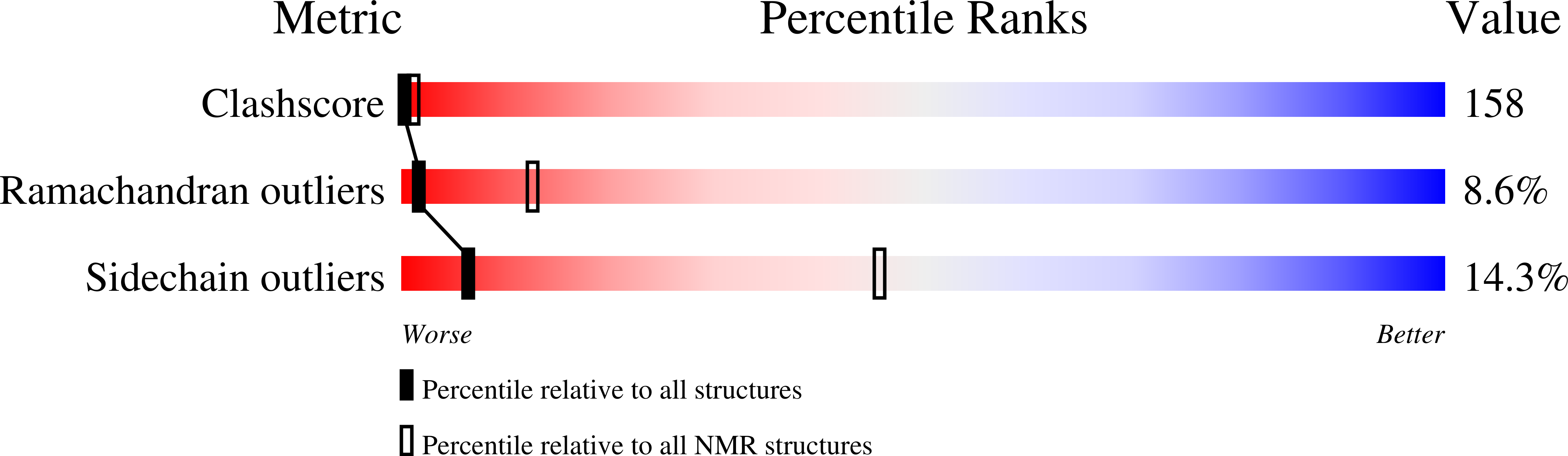
Deposition Date
2000-11-04
Release Date
2001-03-28
Last Version Date
2024-11-06
Entry Detail
PDB ID:
1G6E
Keywords:
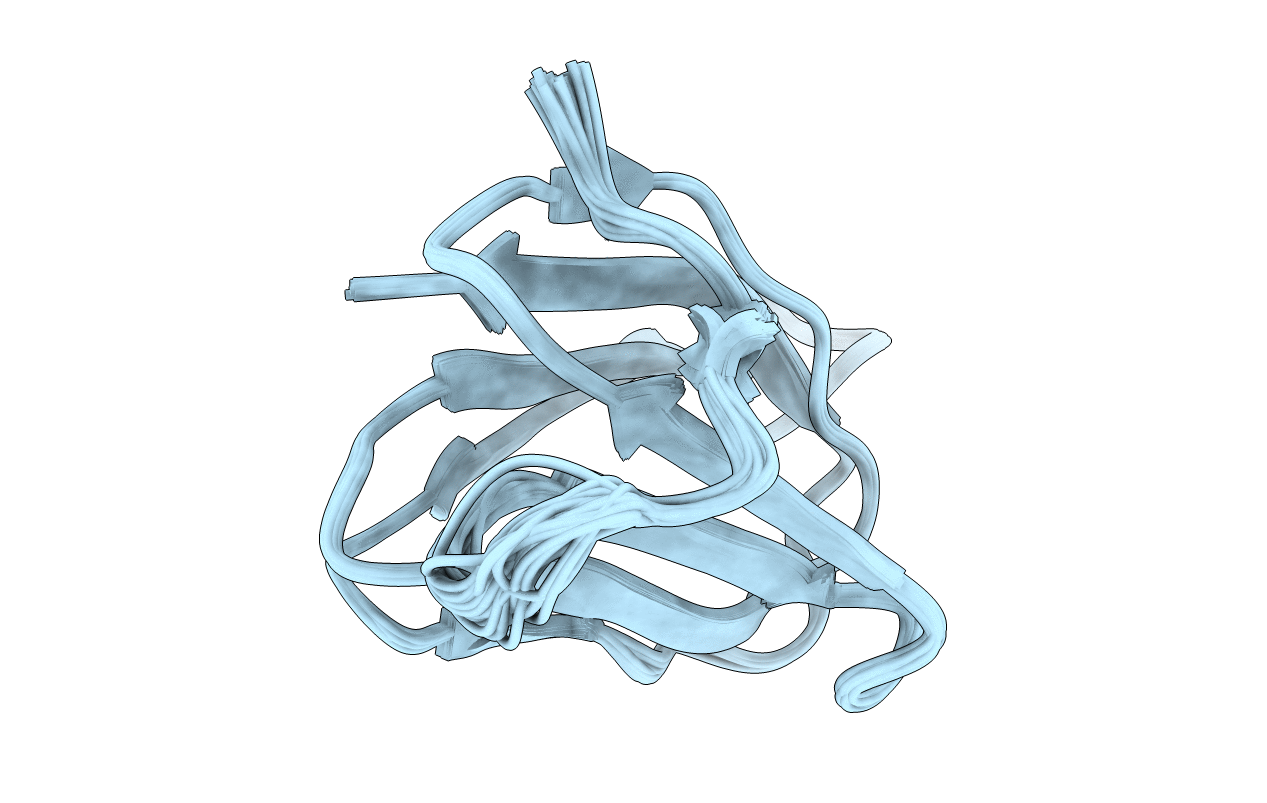
Title:
ANTIFUNGAL PROTEIN FROM STREPTOMYCES TENDAE TU901, 30-CONFORMERS ENSEMBLE
Biological Source:
Source Organism:
Streptomyces tendae (Taxon ID: 1932)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
30
Conformers Submitted:
30
Selection Criteria:
target function