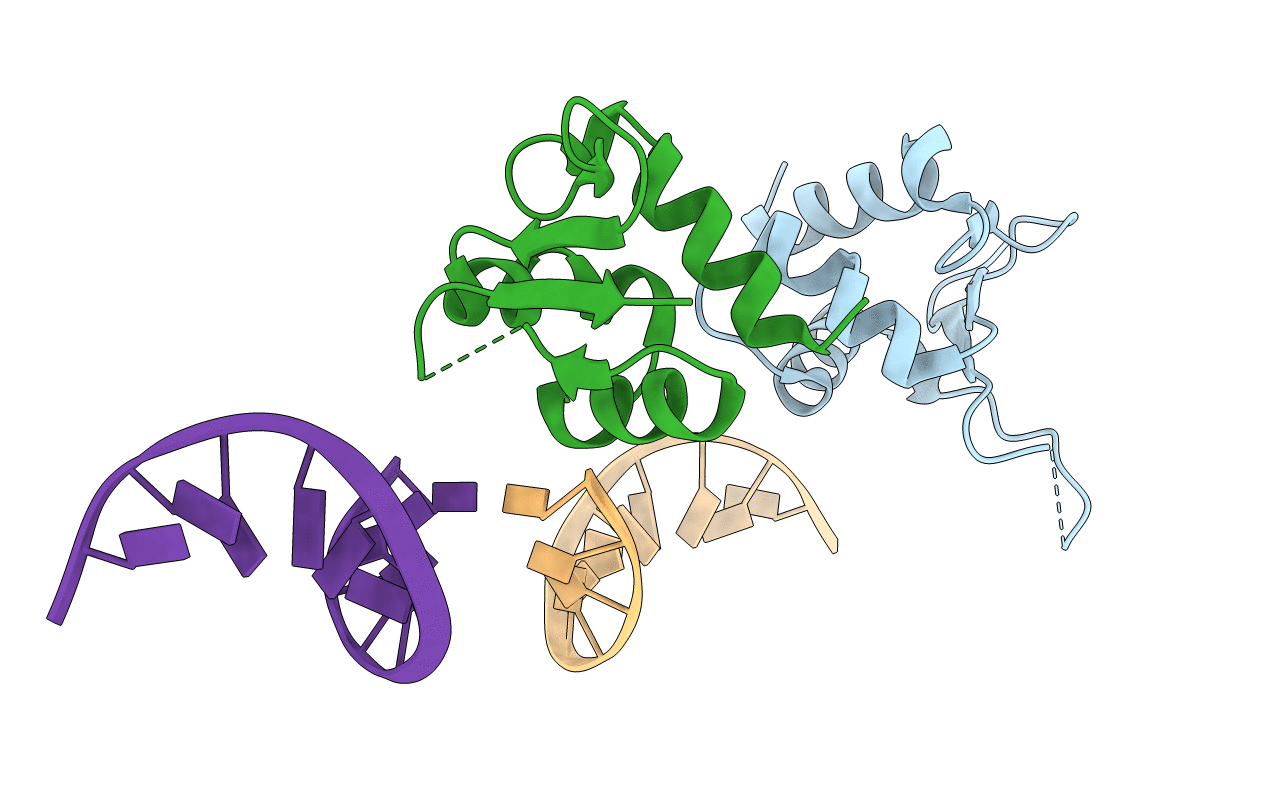
Deposition Date
2000-10-02
Release Date
2001-09-28
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1FYL
Keywords:
Title:
SERENDIPITOUS CRYSTAL STRUCTURE CONTAINING THE HEAT SHOCK TRANSCRIPTION FACTOR'S DNA BINDING DOMAIN AND COGNATE DNA IN A HEAD-TO-HEAD ORIENTATION
Biological Source:
Source Organism(s):
Kluyveromyces lactis (Taxon ID: 28985)
Expression System(s):
Method Details:
Experimental Method:
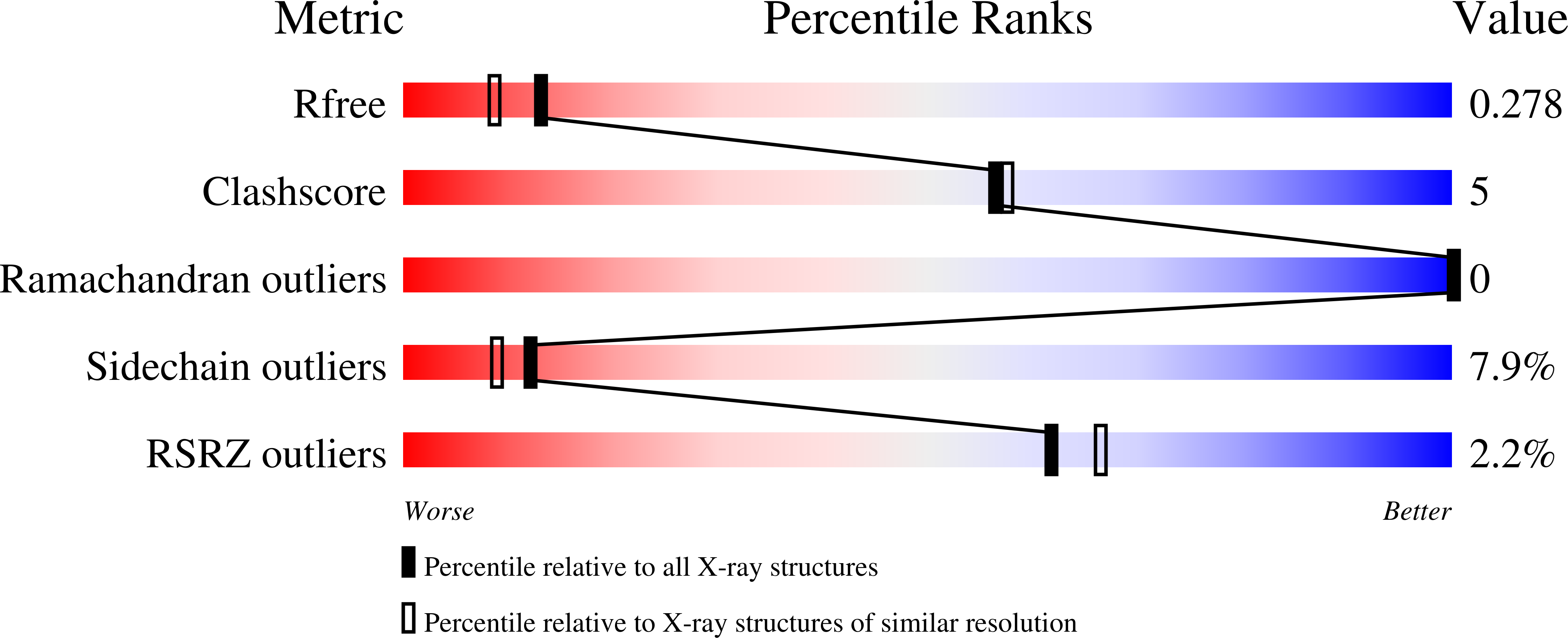
Resolution:
2.10 Å
R-Value Free:
0.3
R-Value Work:
0.21
Space Group:
C 1 2 1