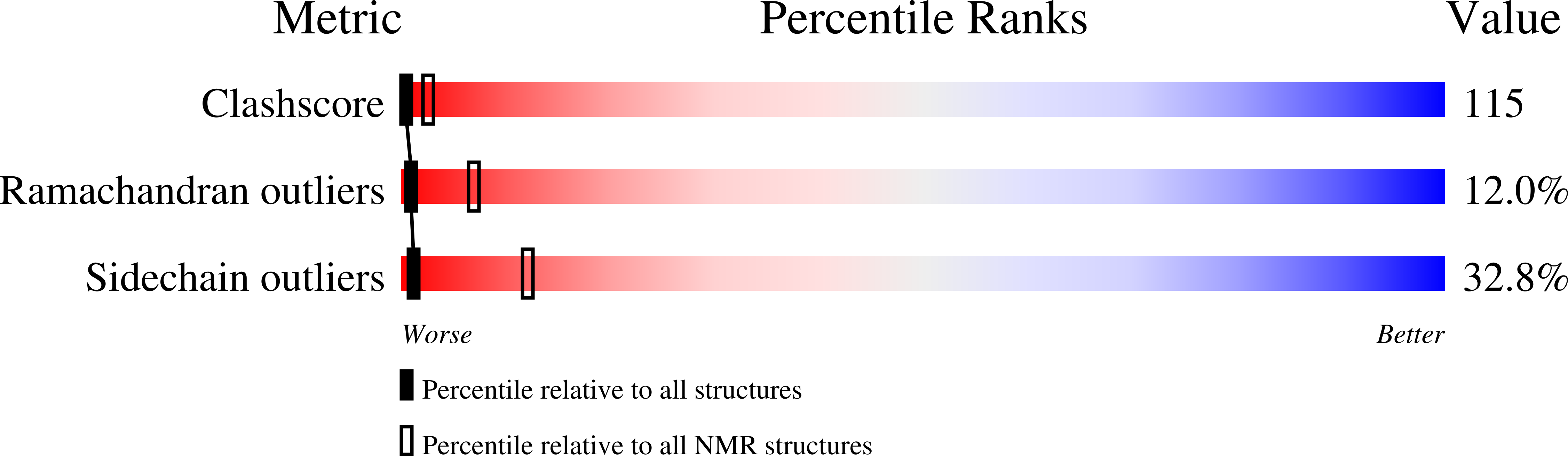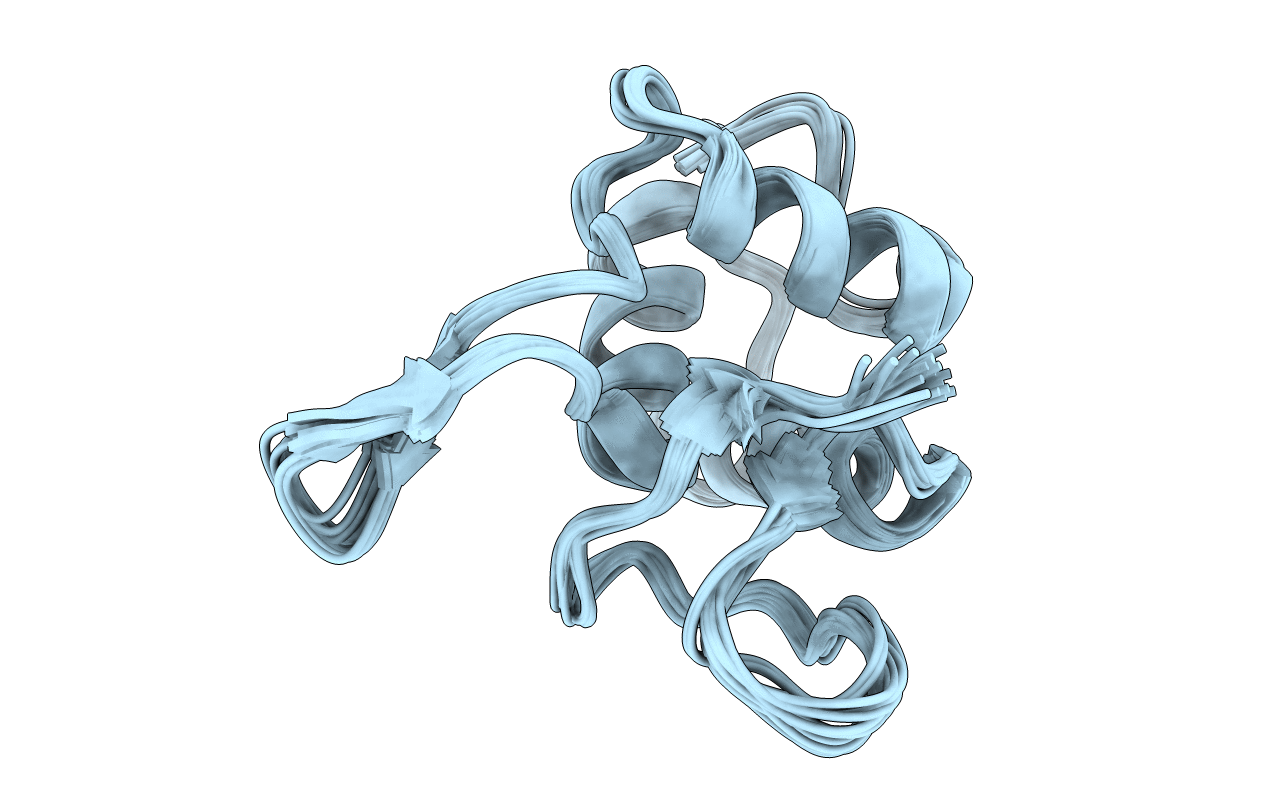
Deposition Date
2000-09-08
Release Date
2001-03-08
Last Version Date
2024-05-29
Entry Detail
PDB ID:
1FSH
Keywords:
Title:
STRUCTURAL BASIS OF THE RECOGNITION OF THE DISHEVELLED DEP DOMAIN IN THE WNT SIGNALING PATHWAY
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
1000
Conformers Submitted:
20
Selection Criteria:
target function