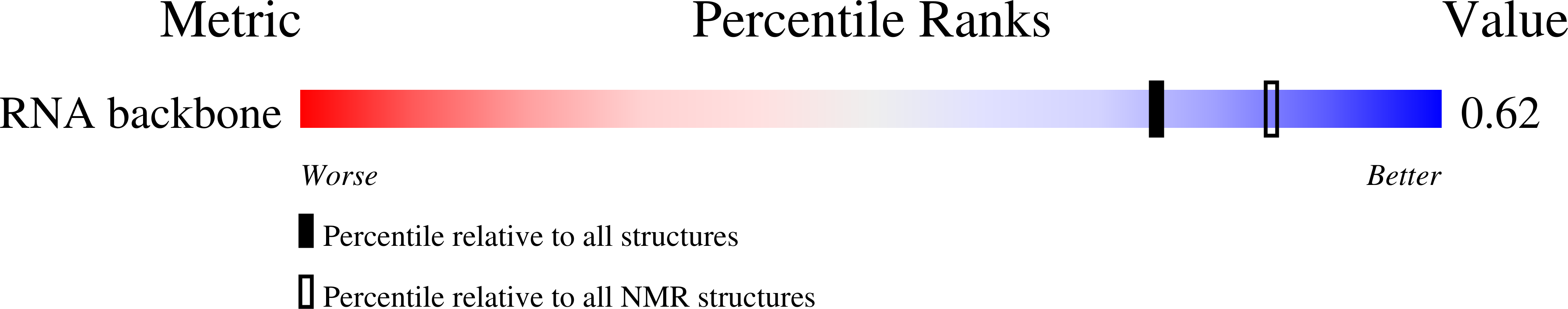
Deposition Date
2000-09-07
Release Date
2001-01-17
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1FQZ
Keywords:
Title:
NMR VALIDATED MODEL OF DOMAIN IIID OF HEPATITIS C VIRUS INTERNAL RIBOSOME ENTRY SITE
Method Details:
Experimental Method:
Conformers Calculated:
1
Conformers Submitted:
1
Selection Criteria:
structures with acceptable covalent geometry, structures with favorable non-bond energy