Deposition Date
1993-10-27
Release Date
1994-01-31
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1FOD
Keywords:
Title:
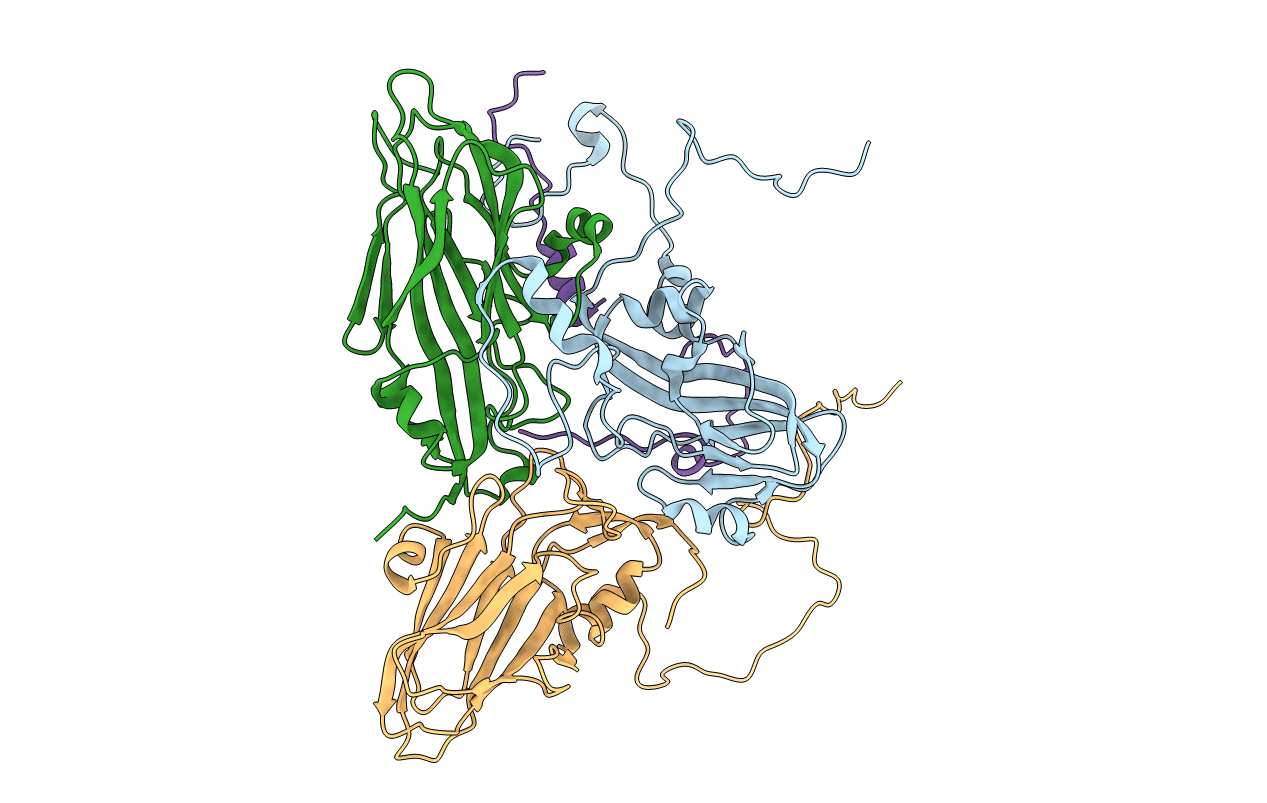
STRUCTURE OF A MAJOR IMMUNOGENIC SITE ON FOOT-AND-MOUTH DISEASE VIRUS
Biological Source:
Source Organism(s):
Foot-and-mouth disease virus (Taxon ID: 12110)
Method Details:
Experimental Method:
Resolution:
2.60 Å
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
I 2 3