Deposition Date
1995-01-05
Release Date
1995-04-20
Last Version Date
2024-02-07
Entry Detail
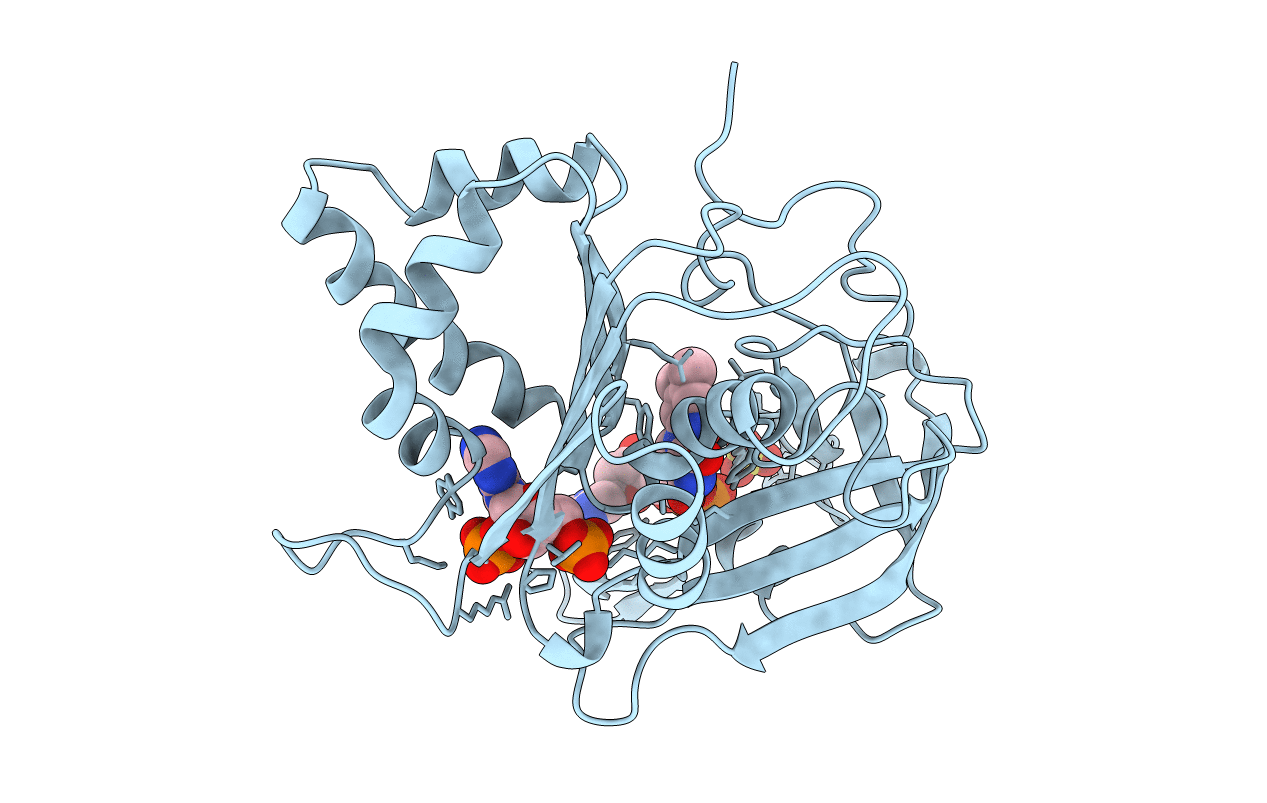
PDB ID:
1FNC
Keywords:
Title:
REFINED CRYSTAL STRUCTURE OF SPINACH FERREDOXIN REDUCTASE AT 1.7 ANGSTROMS RESOLUTION: OXIDIZED, REDUCED, AND 2'-PHOSPHO-5'-AMP BOUND STATES
Biological Source:
Source Organism(s):
Spinacia oleracea (Taxon ID: 3562)
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Observed:
0.14
Space Group:
C 1 2 1


