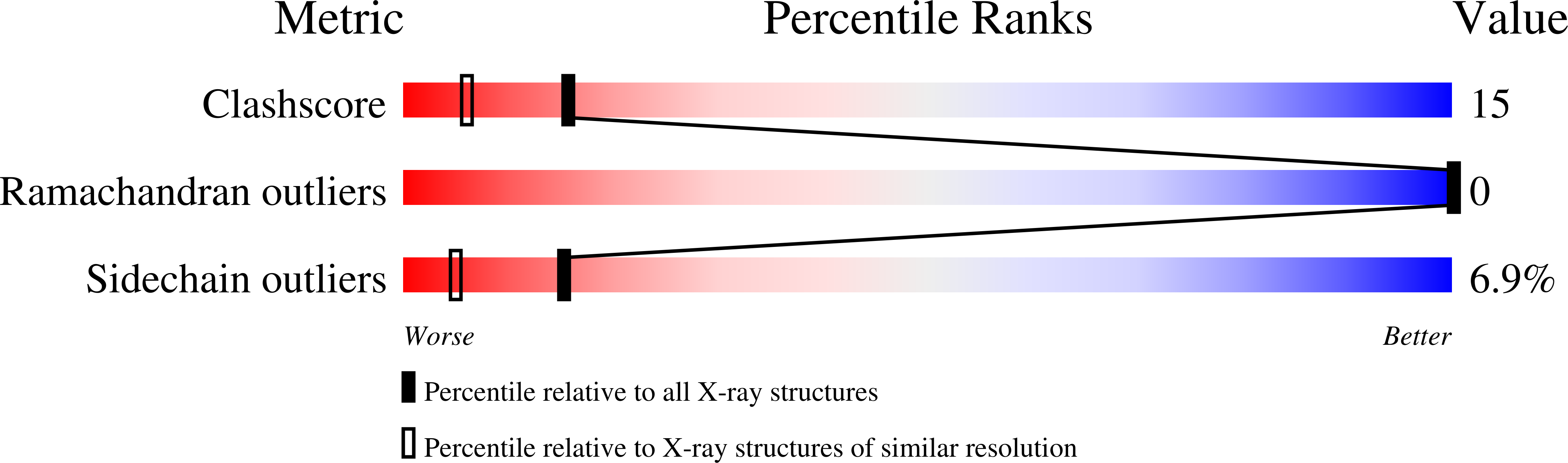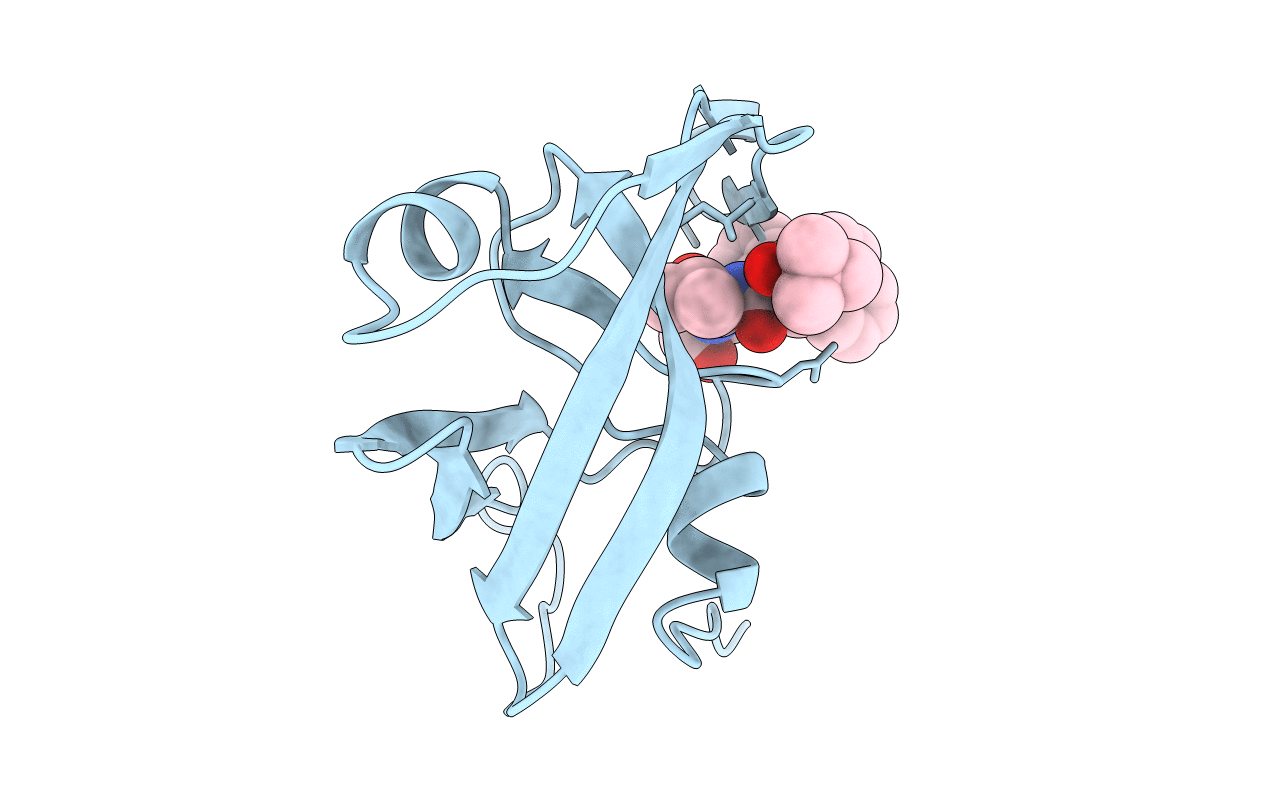
Deposition Date
1996-02-27
Release Date
1996-10-14
Last Version Date
2024-04-03
Entry Detail
PDB ID:
1FMB
Keywords:
Title:
EIAV PROTEASE COMPLEXED WITH THE INHIBITOR HBY-793
Biological Source:
Source Organism(s):
Equine infectious anemia virus (Taxon ID: 11665)
Expression System(s):
Method Details: