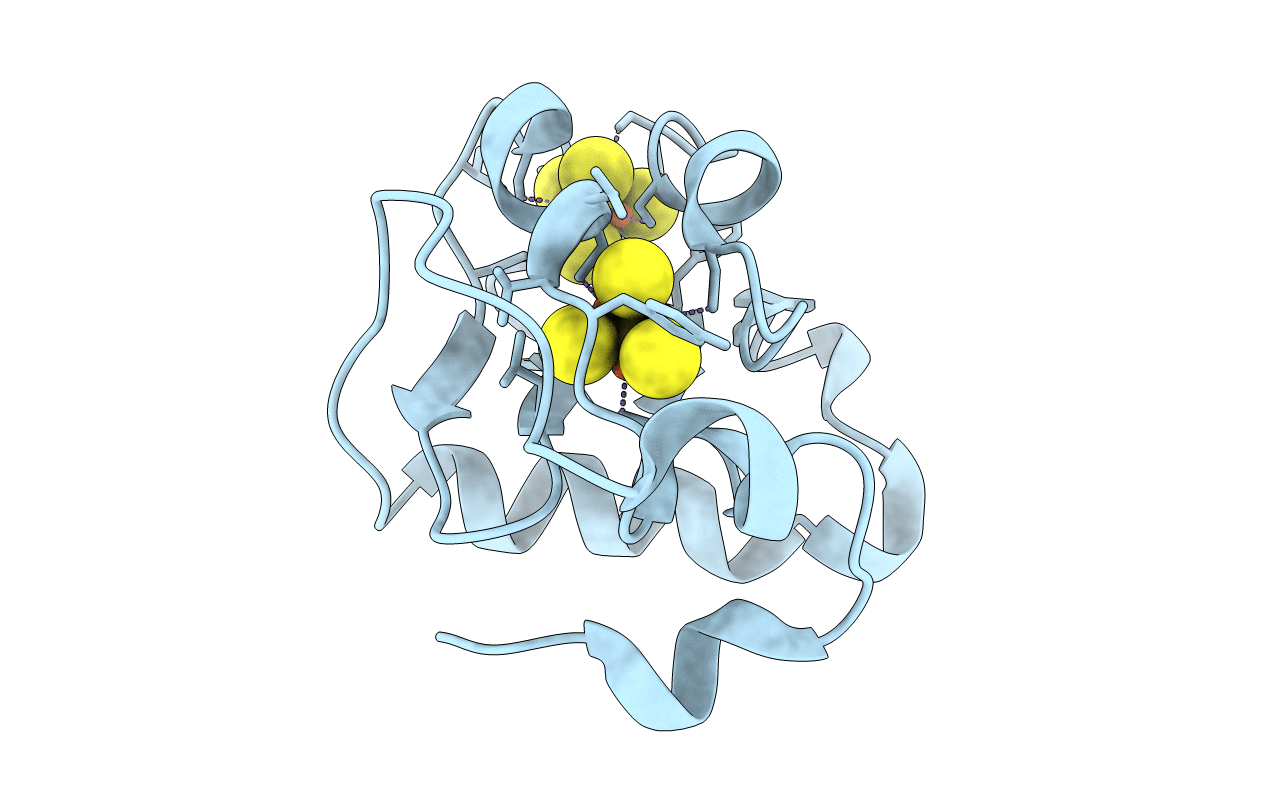Abstact
Crystal structures of Azotobacter vinelandii ferredoxin I (FdI) have been solved and refined at 2.2 to 1.9-A resolution at pH 8 and 6 for both the oxidized and dithionite-reduced proteins. Only the [3Fe-4S] cluster is reduced by dithionite. The four structures (denoted FdI8ox, FdI8red, FdI6ox, and FdI6red) have been compared to address three questions: the effect of reduction at pH 8, the effect of pH change on the structure, and the effect of reduction at pH 6. Comparison of the FdI8ox and FdI8red structures shows that Asp-15 changes conformation in a manner consistent with increased anionic repulsion between this residue and the reduced [3Fe-4S]0 cluster. By revealing an electrostatic interaction between Asp-15 and the [3Fe-4S] cluster, this result supports the conclusion in the accompanying paper (Shen, B., Martin, L. L., Butt, J. N., Armstrong, F. A., Stout, C. D., Jensen, G. M., Stephens, P. J., LaMar, G. N., Gorst, C. M., and Burgess, B. K. (1993) J. Biol. Chem. 268, 25928-25939) that Asp-15 participates in protonation of the reduced [3Fe-4S]0 cluster at acid pH. The [3Fe-4S]0 cluster in the FdI8red structure also displays a distinct shift within the protein as well as internal distortions when compared to the [3Fe-4S]+ cluster in the FdI8ox structure. Comparison of the FdI8ox and FdI6ox structures shows that pH change does not have any significant effect on the [3Fe-4S]+ cluster or surrounding residues. Comparison of the FdI6ox and FdI6red structures shows that reduction at pH 6 also does not have any significant effect on the [3Fe-4S] cluster or Asp-15. The absence of structural change supports the conclusion that at acid pH, the reduced [3Fe-4S] cluster is protonated, i.e. [3Fe-4S]0-H+ (Shen et al., 1993). The cluster is not shifted or distorted as in the FdI8red structure. Instead, the [3Fe-4S]o-H+ cluster FdI8red is structurally similar to the [3Fe-4S]+ cluster (FdI8ox, FdI6ox), which has the same net charge. An Asp-15-Lys-84 salt bridge is observed in all four structures, indicating that Asp-15 is ionized at pH 8 and 6. An ionized state for Asp-15 is also implied by a lack of conformational change in Lys-84; the side chain of this residue rearranges when Asp-15 is substituted with a neutral amino acid (Shen et al., 1993).(ABSTRACT TRUNCATED AT 400 WORDS)