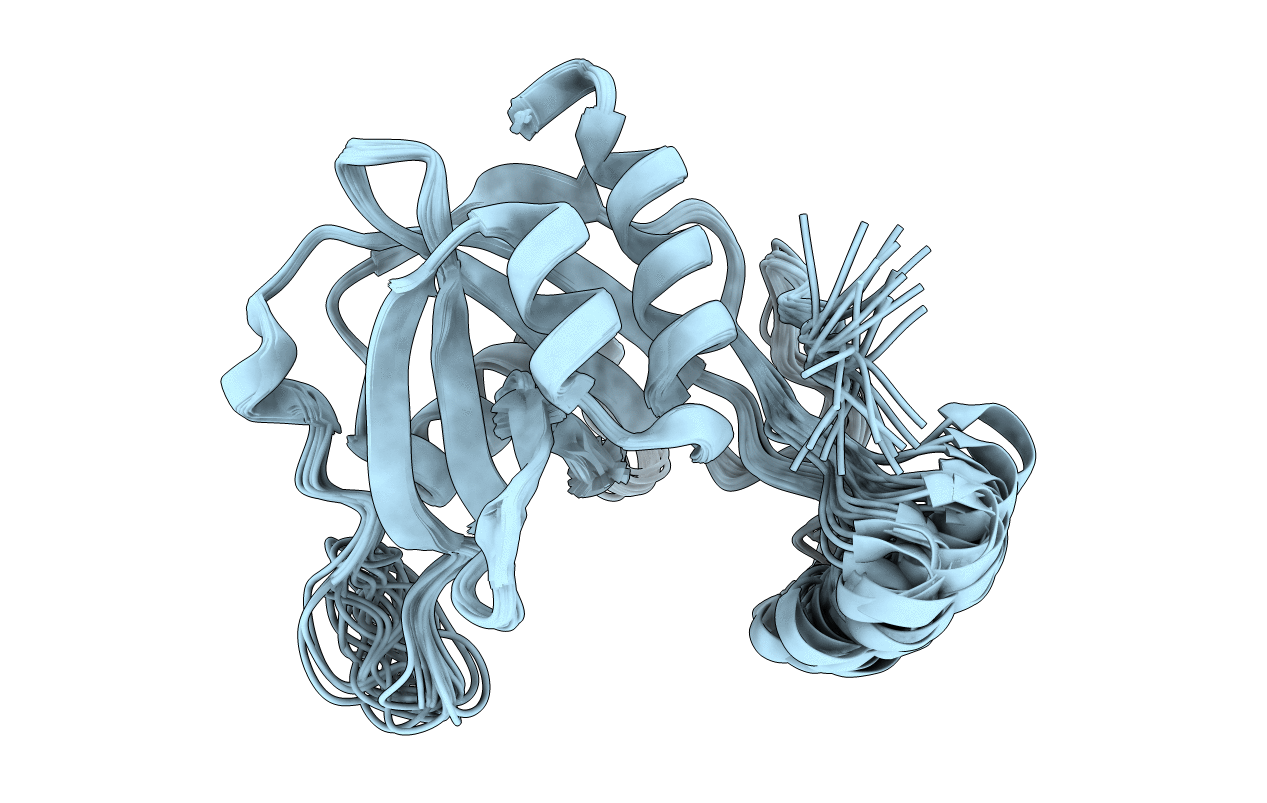
Deposition Date
2000-06-06
Release Date
2001-06-06
Last Version Date
2024-05-29
Entry Detail
PDB ID:
1F3Y
Keywords:
Title:
SOLUTION STRUCTURE OF THE NUDIX ENZYME DIADENOSINE TETRAPHOSPHATE HYDROLASE FROM LUPINUS ANGUSTIFOLIUS L.
Biological Source:
Source Organism(s):
Lupinus angustifolius (Taxon ID: 3871)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
25
Selection Criteria:
structures with favorable non-bond energy,structures with the least restraint violations,target function


