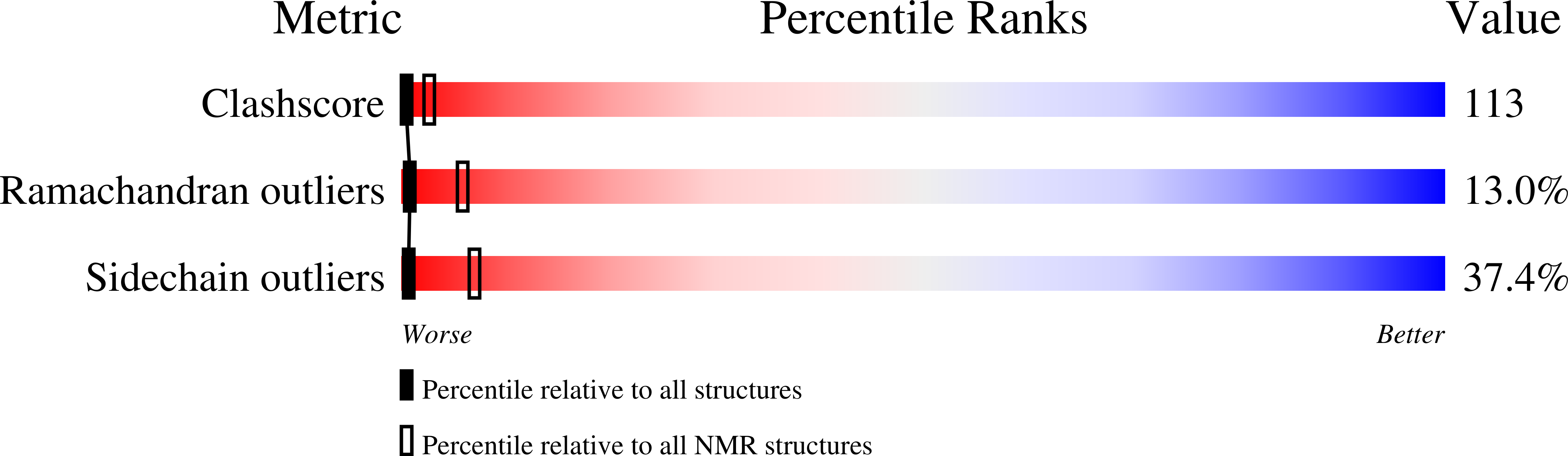
Deposition Date
2000-04-14
Release Date
2001-04-14
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1EUB
Keywords:
Title:
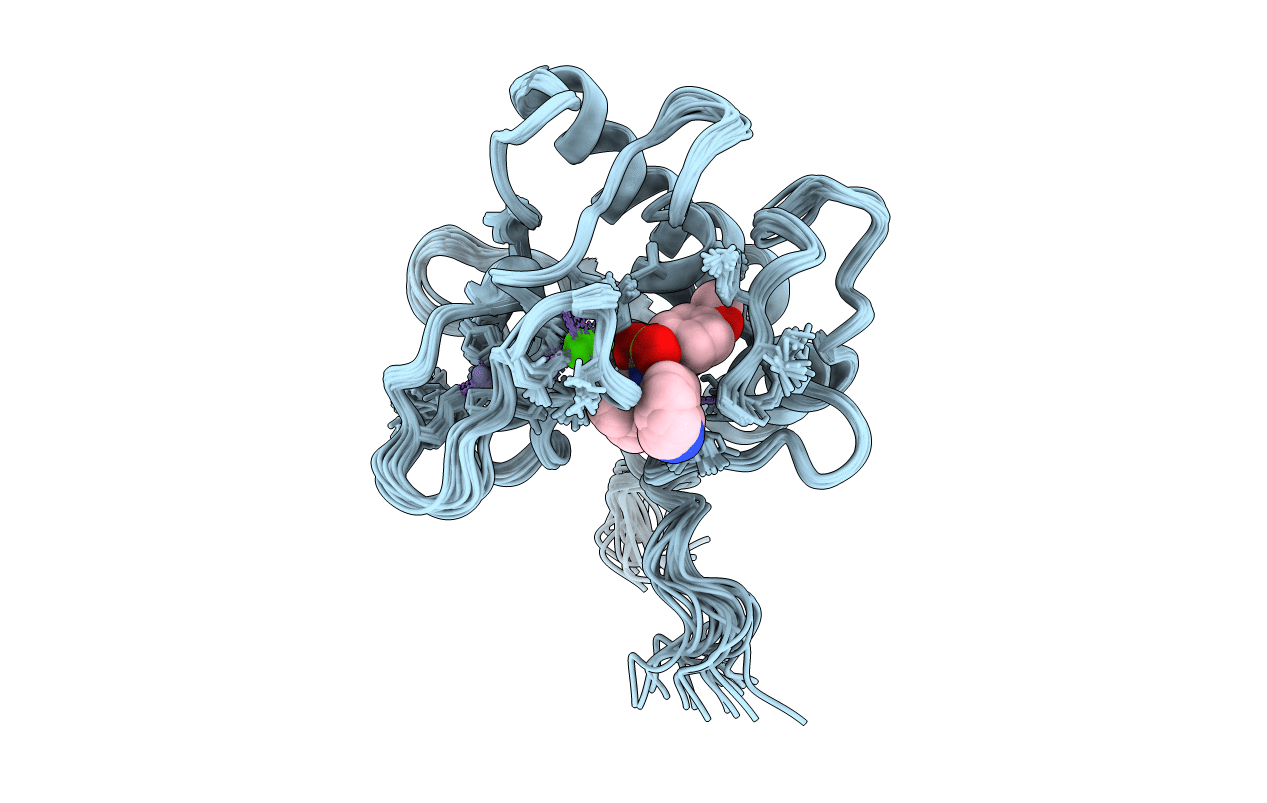
SOLUTION STRUCTURE OF THE CATALYTIC DOMAIN OF HUMAN COLLAGENASE-3 (MMP-13) COMPLEXED TO A POTENT NON-PEPTIDIC SULFONAMIDE INHIBITOR
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
20
Selection Criteria:
BACK CALCULATED DATA AGREE WITH EXPERIMENTAL NOESY SPECTRUM, STRUCTURES WITH ACCEPTABLE COVALENT GEOMETRY,STRUCTURES WITH FAVORABLE NON-BOND ENERGY,TARGET FUNCTION