Deposition Date
2000-04-03
Release Date
2000-04-26
Last Version Date
2024-09-25
Entry Detail
PDB ID:
1EQ8
Keywords:
Title:
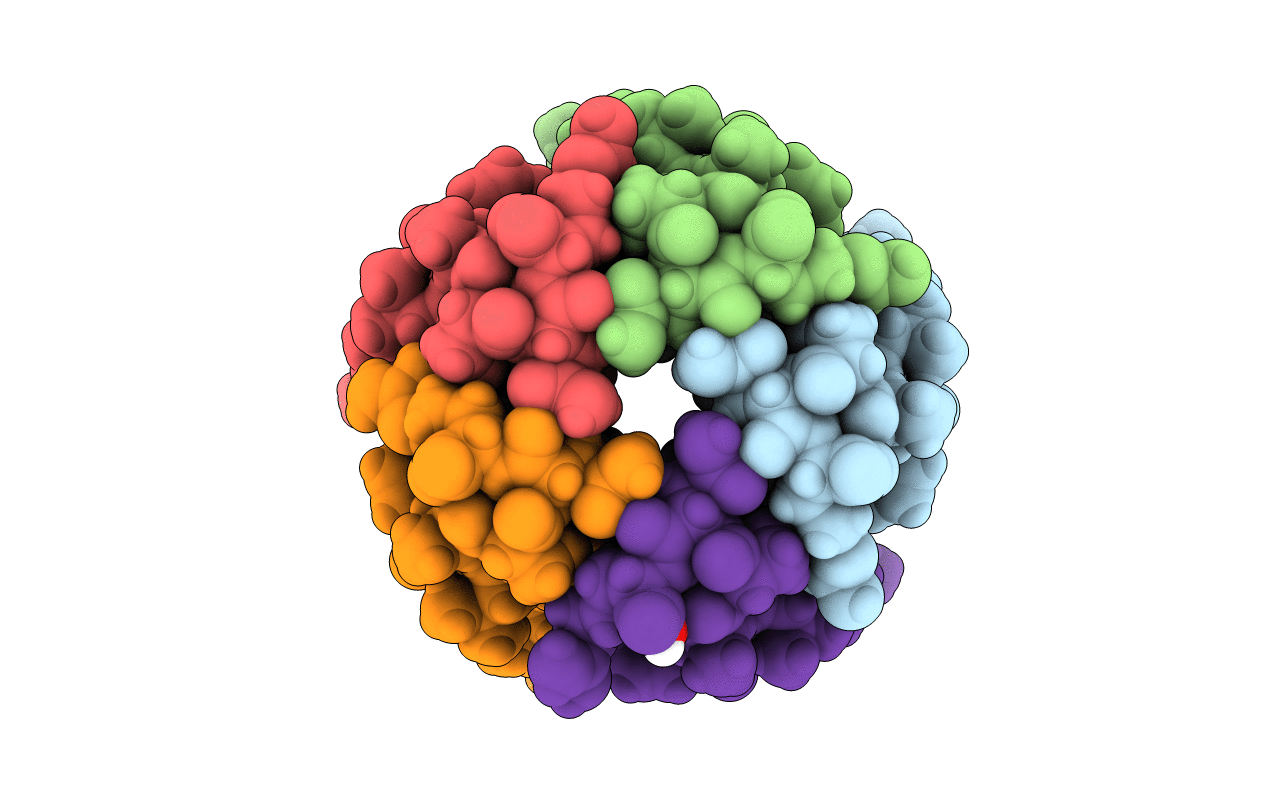
THREE-DIMENSIONAL STRUCTURE OF THE PENTAMERIC HELICAL BUNDLE OF THE ACETYLCHOLINE RECEPTOR M2 TRANSMEMBRANE SEGMENT
Biological Source:
Source Organism(s):
Torpedo californica (Taxon ID: 7787)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
30
Conformers Submitted:
1
Selection Criteria:
structures with favorable non-bond energy