Deposition Date
2000-03-31
Release Date
2002-02-13
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1EQ1
Keywords:
Title:
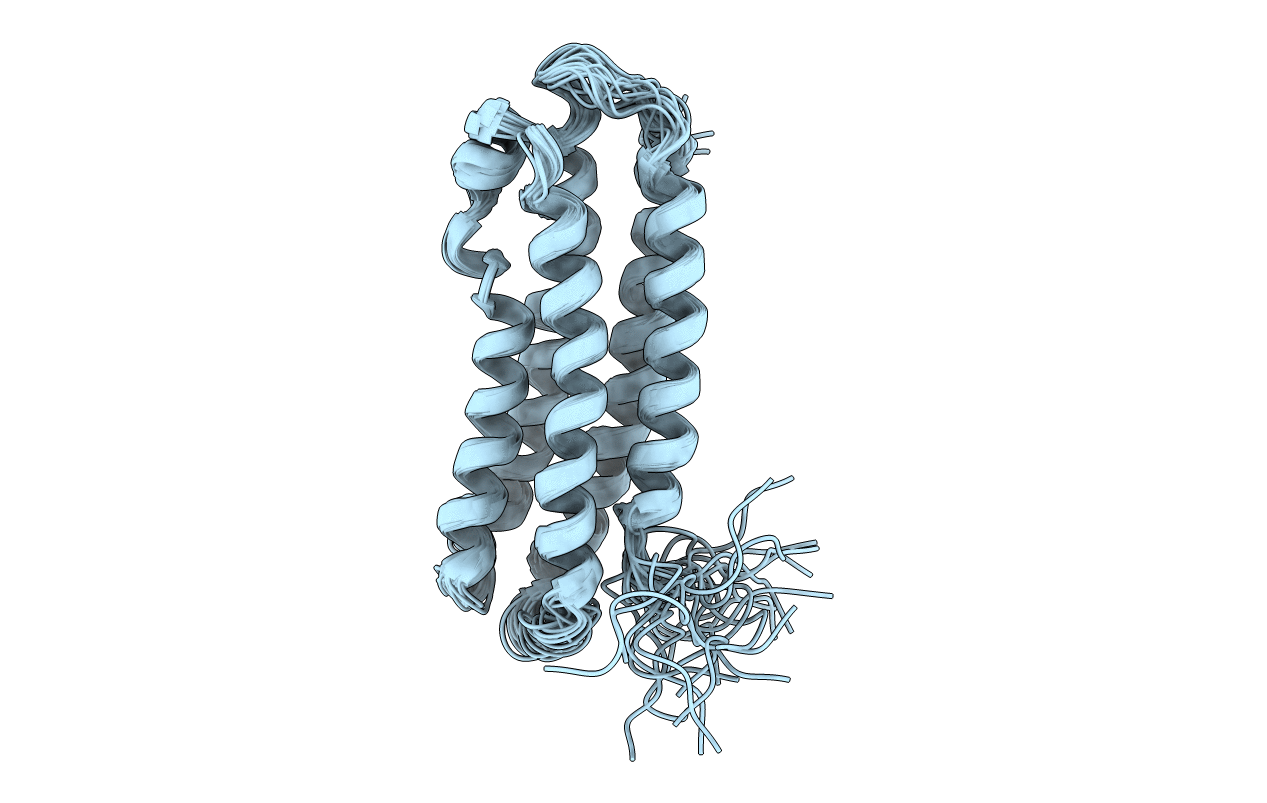
NMR STRUCTURE OF AN EXCHANGEABLE APOLIPOPROTEIN-MANDUCA SEXTA APOLIPOPHORIN-III
Biological Source:
Source Organism:
Manduca sexta (Taxon ID: 7130)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
40
Conformers Submitted:
25
Selection Criteria:
structures with acceptable covalent geometry, structures with favorable
non-bond energy, structures with the least restraint violations, structures
with the lowest energy, target function