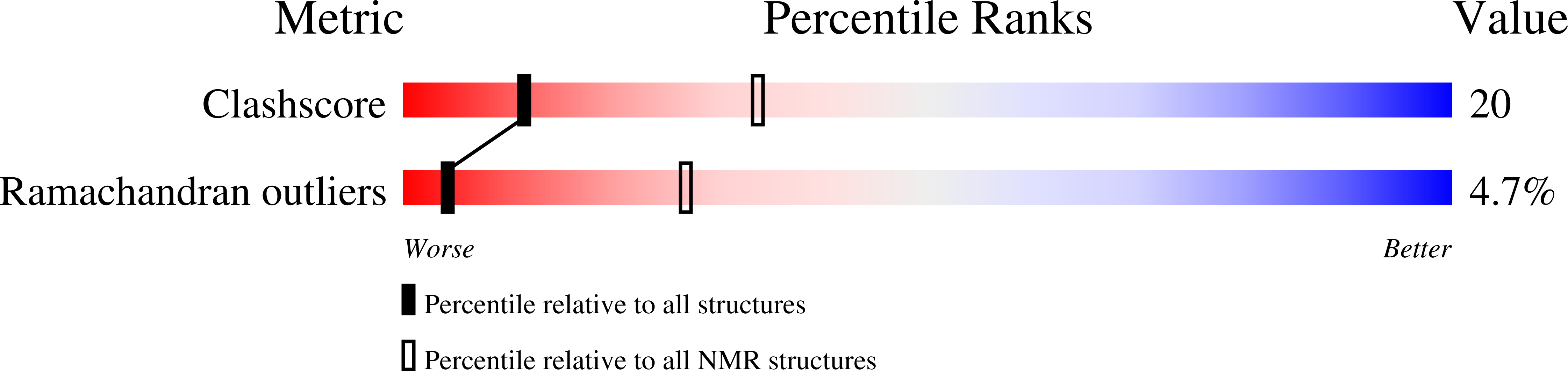
Deposition Date
2000-03-10
Release Date
2000-03-17
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1EKY
Keywords:
Title:
MODEL STRUCTURE FROM NON-NOE BASED NMR STRUCTURE CALCULATION
Biological Source:
Source Organism:
Rhodobacter capsulatus (Taxon ID: 1061)
Method Details:
Experimental Method:
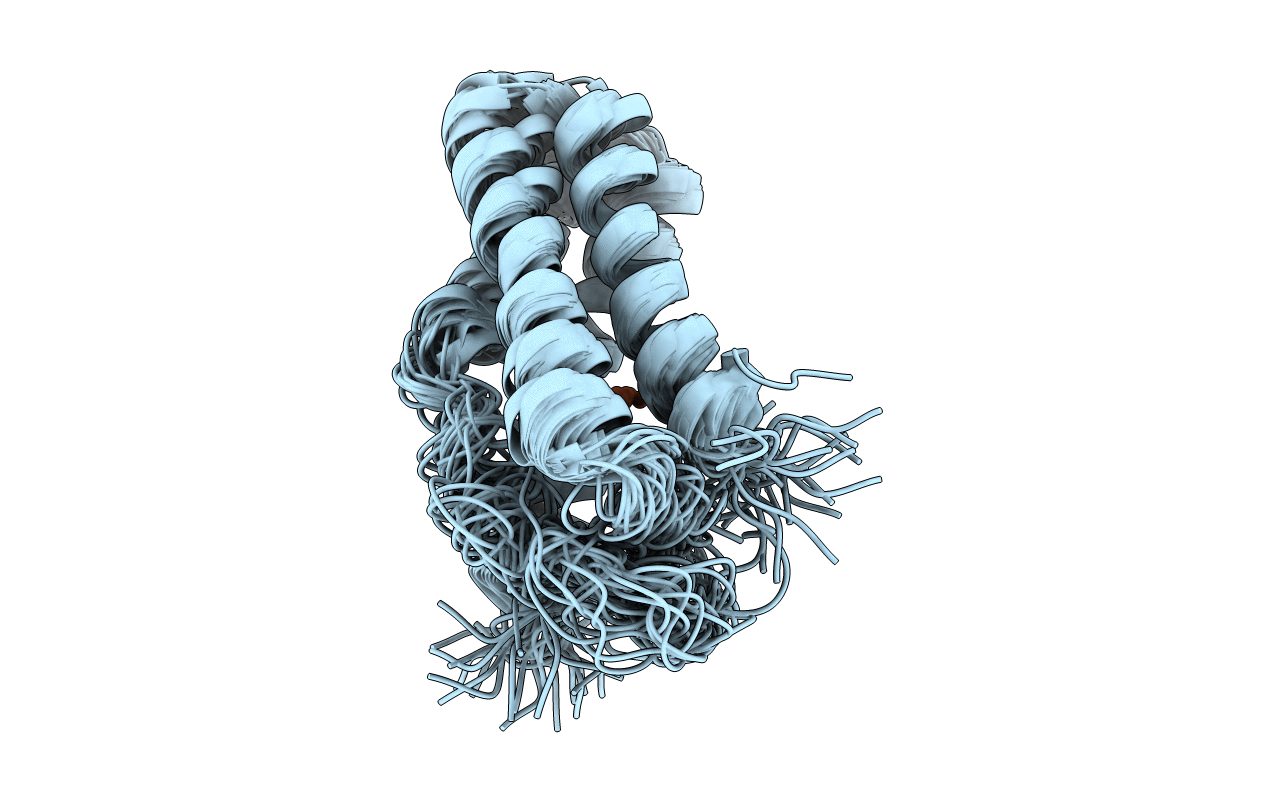
Conformers Calculated:
300
Conformers Submitted:
32
Selection Criteria:
structures with the lowest energy