Deposition Date
2000-03-04
Release Date
2000-09-08
Last Version Date
2021-11-03
Entry Detail
PDB ID:
1EJU
Keywords:
Title:
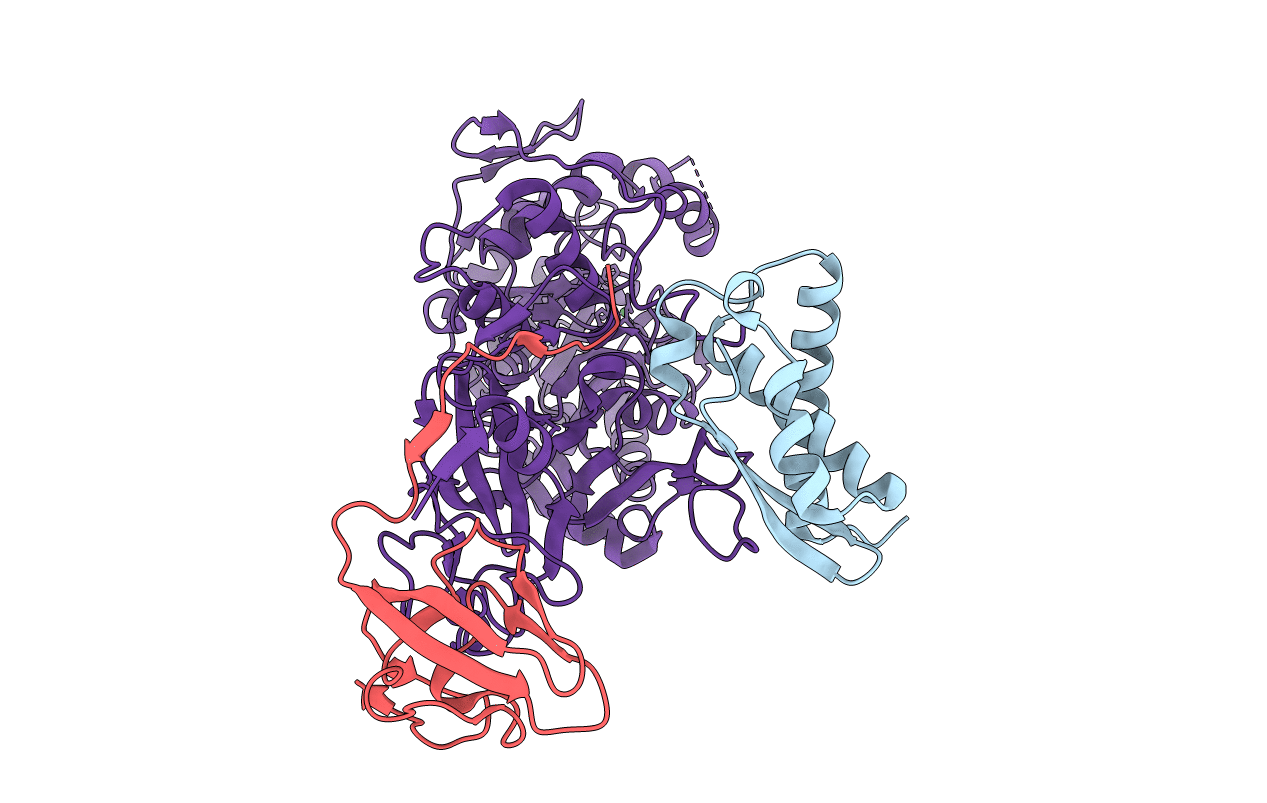
CRYSTAL STRUCTURE OF THE H320N VARIANT OF KLEBSIELLA AEROGENES UREASE
Biological Source:
Source Organism:
Klebsiella aerogenes (Taxon ID: 28451)
Host Organism:
Method Details: