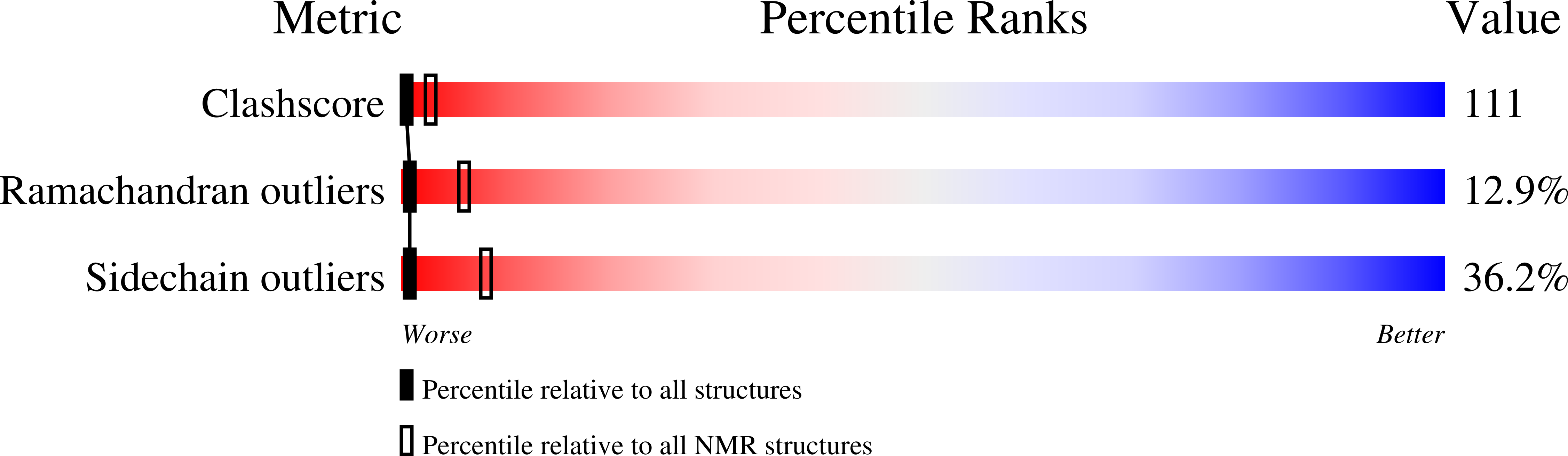Deposition Date
2000-02-23
Release Date
2000-11-17
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1EHX
Keywords:
Title:
NMR SOLUTION STRUCTURE OF THE LAST UNKNOWN MODULE OF THE CELLULOSOMAL SCAFFOLDIN PROTEIN CIPC OF CLOSTRIDUM CELLULOLYTICUM
Biological Source:
Source Organism(s):
Clostridium cellulolyticum (Taxon ID: 1521)
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
20
Selection Criteria:
back calculated data agree with experimental NOESY spectrum, structures with acceptable covalent geometry, structures with favorable non-bond energy, structures with the least restraint violations, structures with the lowest energy