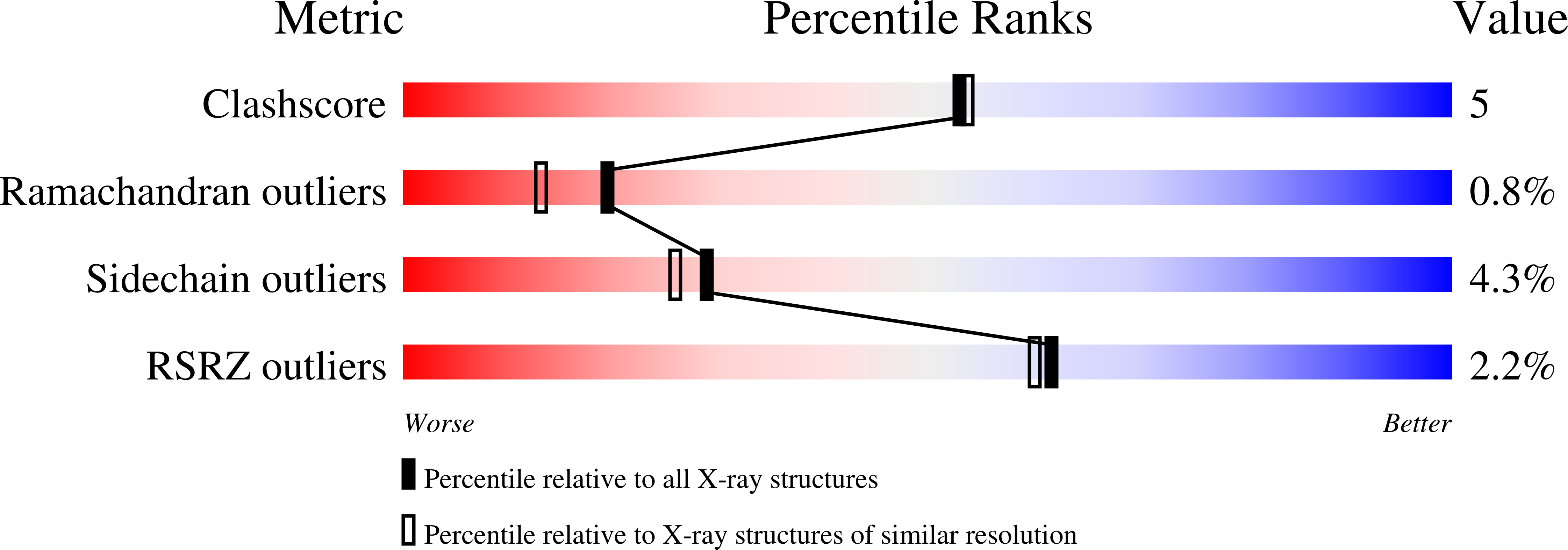
Deposition Date
2000-02-11
Release Date
2000-03-01
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1EFZ
Keywords:
Title:
MUTAGENESIS AND CRYSTALLOGRAPHIC STUDIES OF ZYMOMONAS MOBILIS TRNA-GUANINE TRANSGLYCOSYLASE TO ELUCIDATE THE ROLE OF SERINE 103 FOR ENZYMATIC ACTIVITY
Biological Source:
Source Organism(s):
Zymomonas mobilis (Taxon ID: 542)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Free:
0.19
R-Value Work:
0.16
Space Group:
C 1 2 1