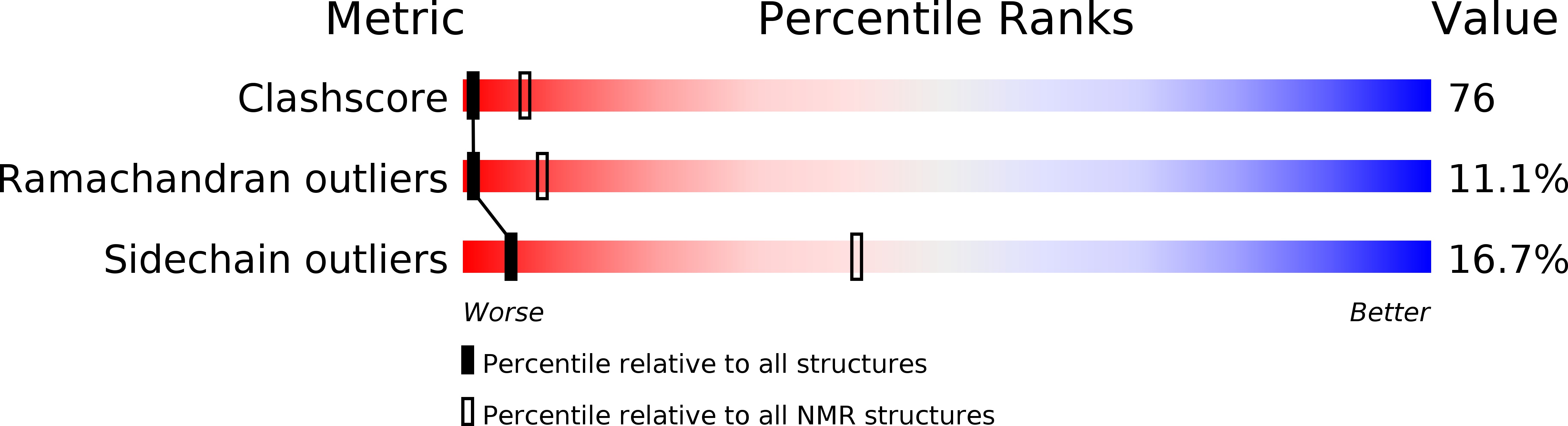
Deposition Date
2000-01-31
Release Date
2000-05-10
Last Version Date
2023-11-15
Entry Detail
PDB ID:
1EE7
Keywords:
Title:
NMR STRUCTURE OF THE PEPTAIBOL CHRYSOSPERMIN C BOUND TO DPC MICELLES
Biological Source:
Source Organism(s):
HYPOMYCES CHRYSOSPERMUS (Taxon ID: 5131)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
1
Selection Criteria:
MINIMIZED DG STRUCTURE, CLOSEST TO AVERAGE STRUCTURE