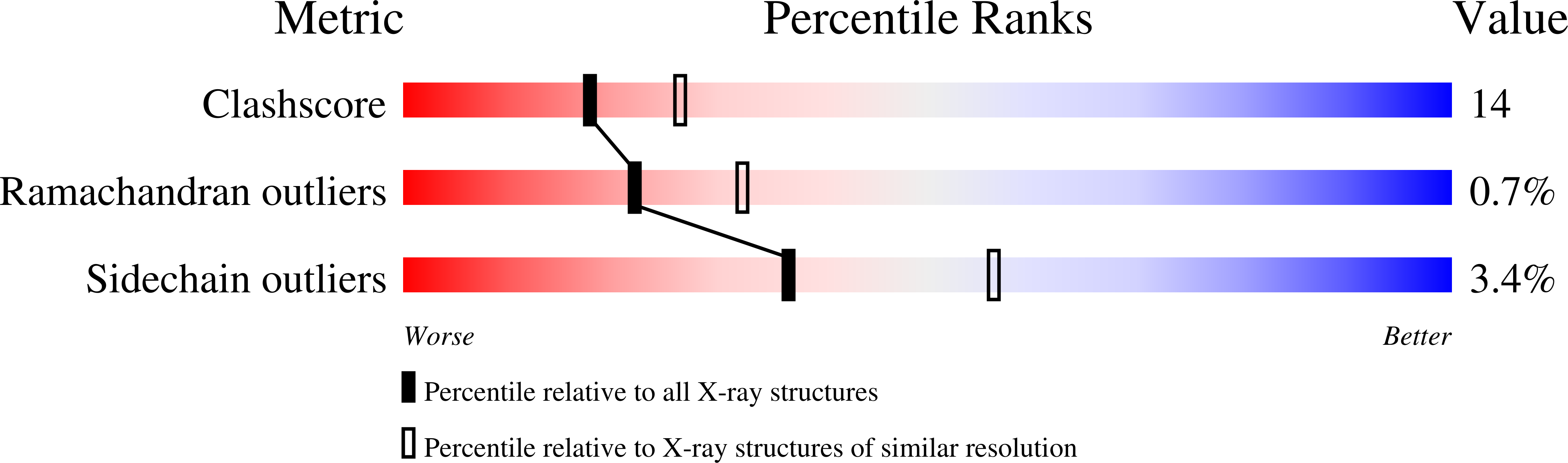
Deposition Date
2000-01-30
Release Date
2000-03-29
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1EE5
Keywords:
Title:
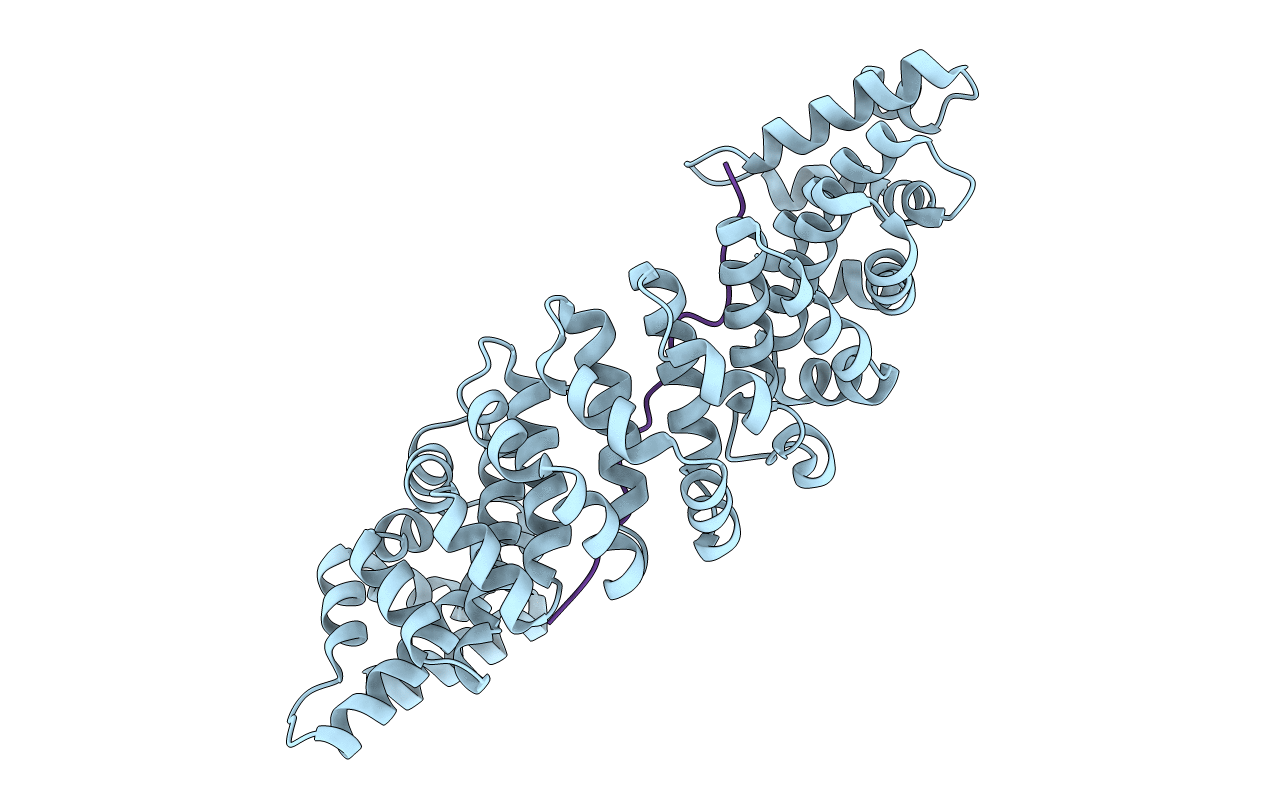
YEAST KARYOPHERIN (IMPORTIN) ALPHA IN A COMPLEX WITH A NUCLEOPLASMIN NLS PEPTIDE
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (Taxon ID: 4932)
Xenopus laevis (Taxon ID: 8355)
Xenopus laevis (Taxon ID: 8355)
Method Details:
Experimental Method:
Resolution:
2.40 Å
R-Value Free:
0.26
R-Value Work:
0.24
Space Group:
C 1 2 1